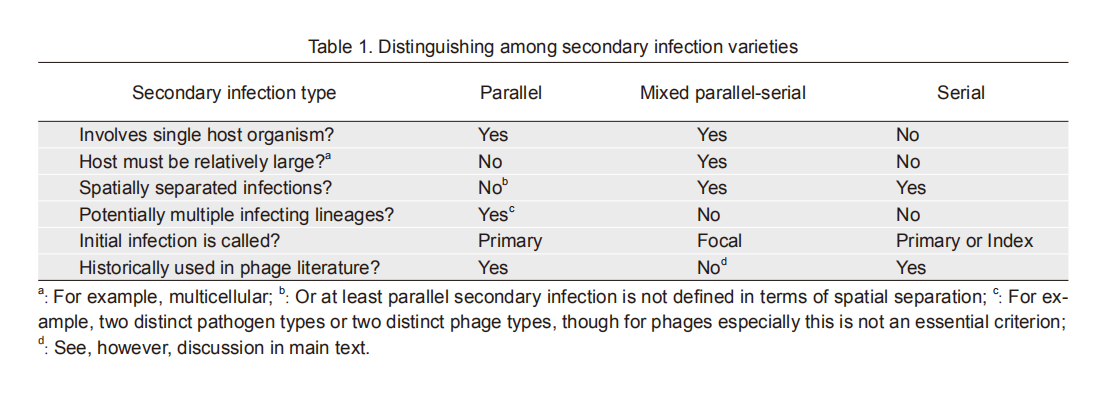
-
Abedon S. 2011. Phage therapy pharmacology: calculating phage dosing. Adv Appl Microbiol. 77:10-40.
-
Abedon ST. 1990. Selection for lysis inhibition in bacteriophage. J Theor Biol. 146:501-511.
doi: 10.1016/S0022-5193(05)80375-3
-
Abedon ST. 1992. Lysis of lysis inhibited bacteriophage T4-infected cells. J Bacteriol. 174:8073-8080.
doi: 10.1128/jb.174.24.8073-8080.1992
-
Abedon ST. 1994. Lysis and the interaction between free phages and infected cells. In: The Molecular Biology of Bacteriophage T4. Karam JD, Kutter E, Carlson K, and Guttman B (eds). Washington DC: ASM Press, pp397-405.
-
Abedon ST. 1999. Bacteriophage T4 resistance to lysis-inhibition collapse. Genet Res, 74:1-11.
doi: 10.1017/S0016672399003833
-
Abedon ST. 2008. Phage population growth: constraints, games, adaptation. In: Bacteriophage Ecology. Abedon ST (ed). Cambridge: Cambridge University Press, pp64-93.
-
Abedon ST. 2009a. Bacteriophage intraspecific cooperation and defection. In: Contemporary Trends in Bacteriophage Research.
-
Adams HT (ed). Hauppauge: Nova Science Publishers, pp191-215.
-
Abedon ST. 2009b. Disambiguating bacteriophage pseudolysogeny: an historical analysis of lysogeny, pseudolysogeny, and the phage carrier state. In: Contemporary Trends in Bacteriophage Research. Adams HT (ed). Hauppauge: Nova Science Publishers, pp285-307.
-
Abedon ST. 2009c. Kinetics of phage-mediated biocontrol of bacteria. Foodborne Pathog Dis, 6:807-815.
doi: 10.1089/fpd.2008.0242
-
Abedon ST. 2011a. Bacteriophages and Biofilms: Ecology, Phage Therapy, Plaques. Hauppauge: Nova Science Publishers.
-
Abedon ST. 2011b. Lysis from without. Bacteriophage. 1: 46-49.
-
Abedon ST. 2012a. Phage therapy best practices. In: Bacteriophages in Health and Disease. Hyman P, Abedon ST (eds). Wallingford: CABI Press, pp256-272.
-
Abedon ST. 2012b. Spatial vulnerability: bacterial arrangements, microcolonies, and biofilms as responses to low rather than high phage densities. Viruses. 4:663-687.
doi: 10.3390/v4050663
-
Abedon ST. 2012c. Thinking about microcolonies as phage targets. Bacteriophage. 2:200-204.
doi: 10.4161/bact.22444
-
Abedon ST. 2014. Bacteriophages as drugs: the pharmacology of phage therapy. In: Phage Therapy: Current Research and Applications. Borysowski J, Miedzybrodzki R, and Górski A (eds). Norfolk: Caister Academic Press, pp69-100.
-
Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. 2011. Phage treatment of human infections. Bacteriophage. 1:66-85.
doi: 10.4161/bact.1.2.15845
-
Abedon ST, Thomas-Abedon C. 2010. Phage therapy pharmacology. Curr Pharm Biotechnol. 11:28-47.
doi: 10.2174/138920110790725410
-
Adams MH. 1959. Bacteriophages. New York: InterScience.
-
Barron BA, Fischetti VA, Zabriskie JB. 1970. Studies of the bacteriophage kinetics of multicellular systems: a statistical model for the estimation of burst size per cell in streptococci. J Appl Bacteriol. 33:436-442.
doi: 10.1111/jam.1970.33.issue-2
-
Benzer S, Hudson W, Weidel W, Delbruck M, Stent GS, Weigle J J, Dulbecco R, Watson JD, Wollman EL. 1950. A syllabus on procedures, facts, and interpretations in phage. Viruses 1950. Delbruck M (ed). Pasadena: California Institute of Technology, pp100-147.
-
Berngruber TW, Weissing FJ, Gandon S. 2010. Superinfection inhibition and the evolution of viral latency. J Virol, 84:10200-10208.
doi: 10.1128/JVI.00865-10
-
Bigwood T, Hudson JA, Billington C. 2009. Influence of host and bacteriophage concentrations on the inactivation of foodborne pathogenic bacteria by two phages. FEMS Microbiol Lett, 291:59-64.
doi: 10.1111/fml.2009.291.issue-1
-
Bull JJ, Regoes RR. 2006. Pharmacodynamics of non-replicating viruses, bacteriocins and lysins. Proc R Soc Lond B Biol Sci, 273:2703-2712.
doi: 10.1098/rspb.2006.3640
-
Chan BK, Abedon ST. 2012. Phage therapy pharmacology: phage cocktails. Adv Appl Microbiol, 78:1-23.
doi: 10.1016/B978-0-12-394805-2.00001-4
-
Chan BK, Abedon ST, Loc-Carrillo C. 2013. Phage cocktails and the future of phage therapy. Future Microbiol, 8:769-783.
doi: 10.2217/fmb.13.47
-
Davis BM, Kimsey HH, Chang W, Waldor MK. 1999. The Vibrio cholerae O139 Calcutta bacteriophage CTXφ is infectious and encodes a novel repressor. J Bacteriol, 181:6779-6787.
-
Doermann AH. 1948. Lysis and lysis inhibition with Escherichia coli bacteriophage. J Bacteriol, 55:257-275.
-
Espeland EM, Lipp EK, Huq A, Colwell RR. 2004. Polylysogeny and prophage induction by secondary infection in Vibrio cholerae. Environ Microbiol, 6:760-763.
doi: 10.1111/emi.2004.6.issue-7
-
Fogg PC, Allison HE, Saunders JR, McCarthy AJ. 2010. Bacteriophage lambda: a paradigm revisited. J Virol, 84:6876-6879.
doi: 10.1128/JVI.02177-09
-
Fogg PCM, Gossage SM, Smith DL, Saunders JR, McCarthy AJ, Allison HE. 2007. Identification of multiple integration sites for Stx-phage φ24B in the Escherichia coli genome, description of a novel integrase and evidence for a functional anti-repressor. Microbiology. 153:4098-4110.
doi: 10.1099/mic.0.2007/011205-0
-
French RC, Graham AF, Lesley SM, van Rooyen CE. 1952. The contribution of phosphorus from T2r+ bacteriophage to progeny. J Bacteriol. 64:597-607.
-
Fukuda E, Kaminska KH, Bujnicki JM, Kobayashi I. 2008. Cell death upon epigenetic genome methylation: a novel function of methyl-specific deoxyribonucleases. Genome Biol. 9:R163.
doi: 10.1186/gb-2008-9-11-r163
-
Golshahi L, Seed KD, Dennis JJ, Finlay WH. 2008. Toward modern inhalational bacteriophage therapy: nebulization of bacteriophages of Burkholderia cepacia complex. J Aerosol Med Pulm Drug Deliv, 21:351-360.
doi: 10.1089/jamp.2008.0701
-
Hatfull GF, Hendrix RW. 2011. Bacteriophages and their genomes. Curr Opin Virol, 1:298-303.
doi: 10.1016/j.coviro.2011.06.009
-
Hendrix RW. 2008. Phage evolution. In: Bacteriophage Ecology. Abedon ST (ed). Cambridge: Cambridge University Press, pp177-194.
-
Hendrix RW, Smith MCM, Burns RN, Ford ME, Hatfull GF. 1999. Evolutionary relationships among diverse bacteriophages and prophages: All the world's a phage. Proc Natl Acad Sci USA. 96:2192-2197.
doi: 10.1073/pnas.96.5.2192
-
Hudson JA, Billington C, Carey-Smith G, Greening G. 2005. Bacteriophages as biocontrol agents in food. J Food Prot, 68:426-437.
doi: 10.4315/0362-028X-68.2.426
-
Hughes KA, Sutherland IW, Jones MV. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology. 144:3039-3047.
doi: 10.1099/00221287-144-11-3039
-
Hyman P, Abedon ST. 2010. Bacteriophage host range and bacterial resistance. Adv Appl Microbiol, 70:217-248.
doi: 10.1016/S0065-2164(10)70007-1
-
Hyman P, Abedon ST. 2012. Smaller fleas: viruses of microorganisms. Scientifica. 2012:734023.
-
Iyer VN, James AP. 1978. Single-cell studies on the carrier state of bacteriophage IKe, a virus specific for conjugative plasmids of the N incompatibility and conjugative group. Can J Microbiol, 24:1595-1601.
doi: 10.1139/m78-255
-
Mann NH. 2003. Phages of the marine cyanobacterial picophytoplankton. FEMS Microbiol Rev, 27:17-34.
doi: 10.1016/S0168-6445(03)00016-0
-
May RM, Anderson RM. 1983. Parasite-host coevolution. In: Coevolution. Futuyma DJ, Slatkin M (eds). Sunderland: Sinauer Associates, pp186-206.
-
Moussa SH, Kuznetsov V, Tran TA, Sacchettini JC, Young R. 2012. Protein determinants of phage T4 lysis inhibition. Protein Sci, 21:571-582.
doi: 10.1002/pro.2042
-
Moussa SH, Lawler JL, Young R. 2014. Genetic dissection of T4 lysis. J Bacteriol, 196:2201-2209.
doi: 10.1128/JB.01548-14
-
Payne RJH, Jansen VAA. 2001. Understanding bacteriophage therapy as a density-dependent kinetic process. J Theor Biol, 208:37-48.
doi: 10.1006/jtbi.2000.2198
-
Payne RJH, Jansen VAA. 2003. Pharmacokinetic principles of bacteriophage therapy. Clin Pharmacokinet, 42:315-325.
doi: 10.2165/00003088-200342040-00002
-
Payne RJH, Phil D, Jansen VAA. 2000. Phage therapy: The peculiar kinetics of self-replicating pharmaceuticals. Clin Pharmacol Ther, 68:225-230.
doi: 10.1067/mcp.2000.109520
-
Pratt JH. 1899. Secondary infection of the skin and subcutaneous tissues by the bacillus typhosus. J Boston Soc Med Sci, 3:170-173.
-
Ryan EM, Gorman SP, Donnelly RF, Gilmore BF. 2011. Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. J Pharm Pharamcol, 63:1253-1264.
doi: 10.1111/j.2042-7158.2011.01324.x
-
Sanders ME. 1987. Bacteriophages of industrial importance. In: Phage Ecology. Goyal SM, Gerba GP, Bitton G (eds). New York: John Wiley & Sons, pp211-244.
-
Slavcev RA, Hayes S. 2002. Rex-centric mutualism. J Bacteriol, 184:857-858.
doi: 10.1128/JB.184.3.857-858.2002
-
Smith DL, Rooks DJ, Fogg PC, Darby AC, Thomson NR, McCarthy AJ, Allison HE. 2012. Comparative genomics of Shiga toxin encoding bacteriophages. BMC Genomics, 13:311.
doi: 10.1186/1471-2164-13-311
-
Stent GS. 1963. Molecular biology of bacterial viruses. San Francisco: WH Freeman and Co.
-
Sturino JM, Klaenhammer TR. 2006. Engineered bacteriophage-defence systems in bioprocessing. Nat Rev Microbiol, 4:395-404.
doi: 10.1038/nrmicro1393
-
Tran TA, Struck DK, Young R. 2005. Periplasmic domains define holin-antiholin interactions in T4 lysis inhibition. J Bacteriol, 187:6631-6640.
doi: 10.1128/JB.187.19.6631-6640.2005
-
Tran TA, Struck DK, Young R. 2007. The T4 RI antiholin has an N-terminal signal anchor release domain that targets it for degradation by DegP. J Bacteriol, 189:7618-7625.
doi: 10.1128/JB.00854-07
-
Turner PE, Duffy S. 2008. Evolutionary ecology of multi-phage infections. In: Bacteriophage Ecology. Abedon ST (ed). Cambridge: Cambridge University Press, pp195-216.
-
Wei W, Krone SM. 2005. Spatial invasion by a mutant pathogen. J Theor Biol, 236:335-348.
doi: 10.1016/j.jtbi.2005.03.016
-
Weinfeld H, Paigen K. 1964. Evidence for a new intermediate state of the viral chromosome during cooperative infection by host-modified lambda phage. Virology, 24:71-83.
doi: 10.1016/0042-6822(64)90149-7
-
Werner ER, Christensen JR. 1969. Infection by bacteriophage P1 and development of host-controlled restriction and modification and of lysogenic immunity. J Virol, 3:363-368.
-
Yamada T, Kawasaki T, Nagata S, Fujiwara A, Usami S, Fujie M. 2007. New bacteriophages that infect the phytopathogen Ralstonia solanacearum. Microbiology.153:2630-2639.
doi: 10.1099/mic.0.2006/001453-0