HTML
-
Rotaviruses(RVs)are leading etiological agents of acute gastroenteritis in humans worldwide; among them, group A rotaviruses(RVAs)have the highest clinical importance. RVAs cause severe diarrhea in infants and children under 5 years of age in developing countries and contribute to approximately 453, 000 childhood deaths annually(Tate et al., 2012). The antigen structures of RVAs include the outer capsid proteins VP7 and VP4, which define the G-and P-types, respectively. To date, 27 G and 37 P genotypes have been identified(Matthijnssens et al., 2011; Trojnar et al., 2013). In humans, 12 G and 15 P types have been reported in RVAs(Matthijnssens et al., 2012), and G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12P[8] are currently the most important genotypes worldwide(Zeller et al., 2012).
Since 2006, two live vaccines, i.e., Rotarix and RotaTeq, have been used worldwide for the prevention of RVA(World Health Organization, 2013). Rotarix is a monovalent vaccine, derived from a human G1P[8] strain; in contrast, RotaTeq is a pentavalent vaccine containing five human-bovine reassortant RV strains, four of which express human G1, G2, G3, and G4, as well as bovine P7[5]. Another live human-bovine reassortant vaccine called Rotavac expressing P1A[8](human) and G6(bovine)has recently been developed in India(World Health Organization, 2014). After implementation of the RV vaccines Rotarix and RotaTeq in childhood immunization programs, hospitalization owing to RV infection has been reduced by over 90% in industrialized and resource-deprived countries. However, the immunogenicity and efficacy of these vaccines were shown to be lower in Africa(Cunliffe et al., 2012; Sow et al., 2012), Asia(Zaman et al., 2010) and Central America(Patel et al., 2009). Additionally, the risk of vaccine-related intussusception(Eng et al., 2012; Kollaritsch et al., 2015; Soares-Weiser et al., 2012), the presence of adventitious agents(Gilliland et al., 2012; Studer et al., 2002), the transmission of vaccine virus strains from vaccinated children to unvaccinated contacts and vaccine-derived disease in immuno-compromised infants(Anderson, 2008; Patel et al., 2010; Yen et al., 2011)have been observed. Moreover, live vaccine-derived viral reassortants, which could lead to the generation of potentially virulent strains, have been detected in stool samples from infants with diarrhea within 2 weeks of vaccination(Payne et al., 2010). RVA strains with antigenic epitopes that differ substantially from the vaccine viruses or with different genotypes can escape the RVA neutralizing-antibody pressure induced by vaccine(Zeller et al., 2012). Therefore, the development of new vaccines that are more safe and efficacious, preferably for parenteral administration, is needed in order to decrease the rates of rotavirus-related morbidity and mortality worldwide.
As an alternative to live vaccines, virus-like particles(VLPs)have been evaluated as recombinant nonreplicating c and idate vaccines(Kushnir et al., 2012). VP6 of RVA is the group antigen protein and the most abundant viral structure protein. Moreover, VP6 can self-assemble VLPs(Li et al., 2014). Earlier studies have shown that RV VP6-specific IgA monoclonal antibodies lack neutralizing activity, but have protective effect(Burns et al., 1996) and can inhibit RV replication(Feng et al., 2002). Recent studies have demonstrated that antibodies targeting RV VP6 possess broad neutralizing activity in vitro and confer protection against diarrhea in mice(Garaicoechea et al., 2008) and neonatal gnotobiotic piglets(Vega et al., 2013). However, while VP6 has been used as a potential candidate antigen, the protection mechanism is not yet clear(Jalilvand et al., 2015). The use of modified VP6 may be valuable for the development of novel RV vaccines and vaccine vectors. Additionally, using modified VP6 as the vector, chimeric proteins carrying VP4 epitopes were shown to elicit protection from RV infection in vitro, and the VP4 epitopes of the chimeric proteins were found to enhance the immunogenicity of the VP6 protein(Teng et al., 2014). Recombinant vaccines expressing chimeric proteins consisting of the group antigen protein VP6 as the vector and foreign neutralizing epitopes may be potential alternatives for circumventing the problems associated with live attenuated vaccines. However, the components of VP7 as the other major antigen protein of RVA have not yet been used in chimeric protein vaccines, and the major neutralizing epitopes of RVA exist mainly on VP7. Therefore, it is essential to investigate the VP7 epitopes of the future chimeric vaccines.
In the present study, we aimed to determine the antigenic properties of VP7 of RVA. Thus, we selected three neutralizing epitopes derived from VP7 of RVA for insertion into the surface of the VP6 protein as the vector(VP6F). Additionally, three chimeric proteins carrying different VP7 epitopes were constructed, and their immunogenicities were evaluated.
-
Fetal rhesus monkey kidney(MA104)cells were maintained in Eagle's minimal essential medium(MEM)supplemented with 10% fetal bovine serum(FBS). The human RV strain Wa(G1P[8]) and the rhesus monkey RV strain SA11(G3P[2])were maintained in our laboratory. RV strains were adapted to grow in cell culture by serial passage in MA104 cells.
-
The neutralizing antigen epitopes were selected from VP7. Their serotype specific neutralization activities were identified using mutant viruses selected for resistance to neutralizing(serotype-specific)monoclonal antibodies, and their fine antigenic positions were confirmed(Dyall-Smith et al., 2012; Taniguchi et al., 1988). In this paper, three epitopes which were derived from the antigen regions A, C, and B on VP7 of human RVA, were studied. The three epitopes designated as G1[142], G2[87], and G4[208] corresponded to amino acid residues 142–152(MKYDQSLKLDM), 208–220(QTTNTATFETVAD), and 87–101(AEAKNEISDDEWENT)of VP7 of human RVA strain Wa(GenBank accession number AAA47342), St3(ABV53296), and TB-Chen(AAV65736), respectively, and were selected to construct chimera vaccines.
Three pairs of specific oligonucleotides, i.e., 244K-G1[142]f(5′-CATGAAATAT-ACCAAAGTCT-AAATTAGATA-GGGTAC-3′) and 244K-G1[142]r(5′-CCATATCTAA-TTAAGACTTT-GTCATATTTC-TGGTAC-3′), 275B-G4[208]f(5′-CTAGGCAAAC-ACAAATACAG-TACTTTTGAA-CAGTTGCTGA-C-3′) and 275B-G4[208]r(5′-CTAGGATCAG-AACTGTTTCA-AAGTAGCTGT-TTTGTCGTTT-C-3′), and 308X-G2[87]f(5′-TCGAGGCAGA-GCTAAAAATG-GATTTCAGAT-ATGAATGGGA-AATACTC-3′) and 308X-G2[87]r(5′-TCGAGAGTAT-TTCCCATTCA-CATCTGAAAT-TCATTTTTAG-TTCTGCC-3′), of the corresponding eiptopes were synthesized and inserted into the Kpn I, Bln I, and Xho I cloning sites at positions I3(244K-G1[142]), I4(275B-G4[208]), and I6(308X-G2[87])on the VP6F vector by means of genetic recombination(Teng et al., 2014). The gene encoding the chimeric VP6F/VP7 epitope was inserted into the plasmid pETL which was derived from pET-3a(Teng et al., 2014). The resulting expression plasmids were designated pETP6F244K-G1[142], pETP6F275B-G4[208], and pETP6F308X-G2[87], respectively. In pETP6F244K-G1[142] epitope G1[142] was inserted at site I3, epitope G4[208] was inserted at site I4 for pETP6F275B-G4[208], and epitope G2[87] inserted at site I6 for pETP6F308X-G2[87](Figure 1). The above recombinant plasmids were confirmed by restriction endonuclease digestion and nucleotide sequencing(data note shown).
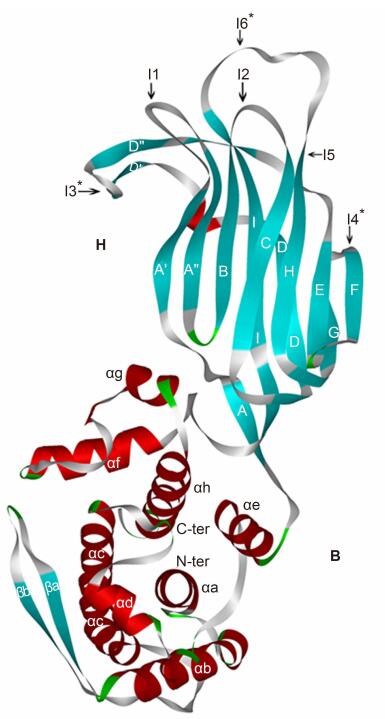
Figure 1. Architecture of the VP6F vector protein by homologous reconstruction with six foreign epitope insertion sites on the outer surface; these sites could be used for insertion of foreign epitopes.Sites I3*, I4*, and I6*were chosen for insertion of epitopes G1[142], G4[208], and G2[87], which were derived respectively from the VP7 of human strains Wa, St3, and TB-Chen in the present study.
-
The pETP6F244K-G1[142], pETP6F275B-G4[208] and pETP6F308X-G2[87] plasmids were transformed separately into E. coli BL21(DE3)competent cells for expression. The transformed E. coli cells were cultured in LB medium supplemented with 200 μg/mL ampicillin, at 37 ℃. After sonication and clarification of the cultured cells, the target proteins were extracted, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDS-PAGE)on 10% gels(Figure 2A), and subjected to western blotting(Figure 3). The expressed proteins were purified as previously described(Teng et al., 2014), and the protein concentrations were determined using Lowry's method(Lowry et al., 1951).
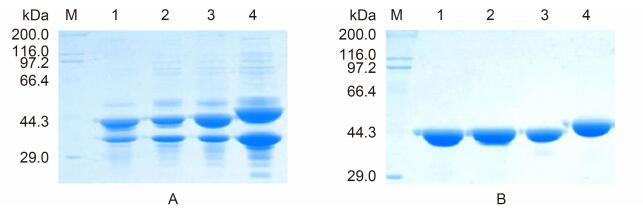
Figure 2. SDS-PAGE (10%) of the expressed chimeric proteins before (A) and after purification (B).Lane M:Molecular weight standard (kDa); lane 1:recombinant vector protein VP6F;lane 2:chimeric proteins 6F/G1[142];lane 3: 6F/G4[208];lane 4:6F/G2[87].
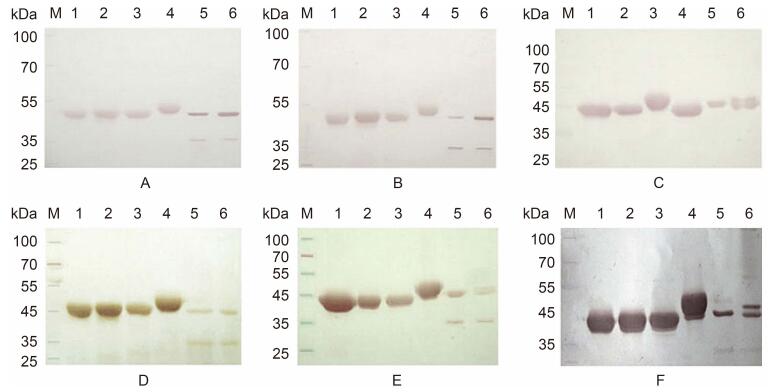
Figure 3. Western blotting of chimeric proteins with antibodies against RV SA11(A), Wa (B), rVP6F (C), 6F/G1[142] (D), 6F/G4[208](E), and 6F/G2[87](F).Protein molecular weight standard (M), vector protein VP6F (1), chimeric proteins 6F/G1[142](2), 6F/G4[208](3), 6F/G2[87](4), SA11(5), and Wa (6).Lanes 1-4 contained 4 μg protein, and lanes 5 and 6 contained 1 × 108.5 of the TCID 50 of RV.
-
The vector protein VP6F and chimeric proteins carrying the VP7 epitope, i.e., G1[142], G2[87], and G4[208] were purified(Figure 2B) and used to immunize guinea pigs. Five-to six-week-old naive guinea pigs were purchased from China Medical Primates Center(Kunming, China); all were negative for RVA infection. Guinea pigs were divided into five groups and housed in biosafety level 2 animal facilities. All animal experiments complied with national and institutional guidelines established by the Institute of Medical Biology Animal Care and Use Ethic Committee, and approved by the Institute of Medical Biology Animal Care and Use Ethic Committee.
The vector protein rVP6F and chimeric proteins, reference Wa and SA11 strains of RV were used as immunogens(n = 4 guinea pigs per immunogen). For inoculation with rVP6F, G1[142], G4[208], and G2[87], guinea pigs in each group were inoculated subcutaneously in the hind leg with 120 μg of each immunogen in 100 μL of TNS solution(15 mmol/L Tris, 150 mmol/L NaCl, pH 7.0)in the absence of any adjuvant. For Wa and SA11 inoculation, 1 × 107 of the 50% tissue culture infective dose(TCID50)of the virus was used for each animal at each injection. Four guinea pigs were used as negative controls and were mock-inoculated with 100 μL of TNS solution. Each animal was inoculated three times(at 0, 14 and 28 days). Serum samples were collected individually from each guinea pig at the fifth day after the last inoculation, and antibodies were detected by western blotting and neutralization tests.
-
Expressed recombinant proteins including chimeric proteins(about 4 µg each)were subjected to SDS-PAGE. Samples were suspended in gel loading buffer(2 mmol/L EDTA, 50 mmol/L Tris, pH 6.8, 10% glycerol, 1% SDS, 1% β-mercaptoethanol, 0.05% bromophenol blue), heated(100 ℃, 5 min), and subjected to SDS-PAGE on 10% gels. Separated proteins were transferred to polyvinylidene fluoride(PVDF)membranes, incubated with antibodies from immunized guinea pig serum(at a 1:400 dilution), and subsequently incubated with horseradish peroxidase conjugated goat anti-guinea pig immunoglobulin G(IgG; 1:2, 000 dilution; Sigma, St. Louis, MO, USA). The target protein bands were finally visualized by incubation with 3, 3′-diaminobenzidine tetrahydrochloride(DAB; Sigma).
-
Immunofluorescence was performed to detect RV antigens in virus infected cells. Briefly, MA104 cell monolayers on glass coverslip cultured in MEM were washed twice with PBS and infected with SA11 or Wa pre-treated with acetylated trypsin. The coverslips were removed 12 h after infection, washed twice with PBS, fixed with prechilled methanol, and rehydrated for 10 min at 4 ℃ with 70%, 30%, and 10% prechilled ethanol. After washing with PBS, coverslips were incubated for 1 h at 37 ℃ with antiserum(1:400 dilution in 0.1% of bovine serum albumin)collected from guinea pigs inoculated with VP6F, chimeric proteins, SA11, or Wa; serum collected from pre-immune guinea pigs; or serum collected from mock inoculated guinea pigs. After washing, coverslips were incubated at 37 ℃ for 1 h with goat anti-guinea pig IgG labeled with FITC(1:100 dilution), and fluorescence was detected under an ultraviolet microscope.
-
The RV strains Wa and SA11 were used to estimate the neutralizing reactivity of antibodies elicited in the serum of guinea pigs inoculated with the chimeric proteins as previously reported(Teng et al., 2014). Briefly, 50 μL of acetylated trypsin treated virus solution containing 100 of the TCID50 of the virus was mixed with an equal volume of the guinea pig antiserum at 2-fold serial dilutions and then incubated for 1 h at 37 ℃. The mixture was then added to MA104 cell monolayers in 96-well plates. Each dilution was determined with four wells. The cell plates were incubated at atmosphere containing 5% CO2 for 48 h, and the cytopathic effect(CPE)was observed. Neutralizing titers were defined as the highest dilution of antiserum that protected 50% of cells from virus infection.
Cells and viruses
VP7 epitopes and construction of recombinant plasmids
Protein expression and detection
Animal immunization
Western blotting
Immunofluorescence
Determination of neutralizing antibody reactivity
-
The vector protein VP6F and chimeric proteins harboring VP7 epitopes were highly expressed(Figure 2A). Expressed VP6F and the chimeric protein 6F/G1[142](carrying the epitope G1[142]), 6F/G4[208](carrying the epitope G4[208]), and 6F/G2[87](carrying the epitopeG2[87])had molecular weights of 43.2, 44.7, 44.8, and 45.2 kDa, as expected, and accounted for 46.7%, 45.2%, 47.0%, and 51.0% of the total proteins of the transformed cells, respectively(Figure 2A). The chimeric proteins were purified(Figure 2B) and used to immunize guinea pigs.
-
The antigenicity of the chimeric proteins was detected by Western blotting. The results showed that all of the chimeric proteins could be specifically recognized by antibodies in the sera derived from guinea pigs inoculated with SA11(Figure 3A), Wa(Figure 3B)or recombinant VP6F(Figure 3C). There was no immunoreactivity to serum derived from pre-immune or negative control animals(data not shown). Antibodies from the guinea pigs that were immunized with chimeric proteins carrying a single VP7 epitope reacted with the vector protein VP6F, chimeric proteins, and VP6(about 45 kDa) and VP7(37 kDa)of RV SA11 and Wa(Figure 3D–3F). No immunoreactivity of serum derived from pre-immune or negative control animals was observed(data not shown).
-
RV antigens in SA11-or Wa-infected MA104 cells were detected by immunofluorescence assay using guinea pig antibodies targeting the chimeric proteins(Figure 4). The results showed that RV antigen in SA11-or Wa-infected MA104 cells could be specifically detected by antibodies targeting the vector protein VP6F; chimeric proteins 6F/G1[142], 6F/G2[87], and 6F/G4[208], RV SA11, Wa, and antiserum derived from guinea pigs mock inoculated with PBS. As control, no fluorescence was detected in SA11-or Wa-infected cells when detected with negative control or pre-inoculation sera(data not shown). No fluorescence was detected in uninfected cells for any of the above antibodies(data not shown).
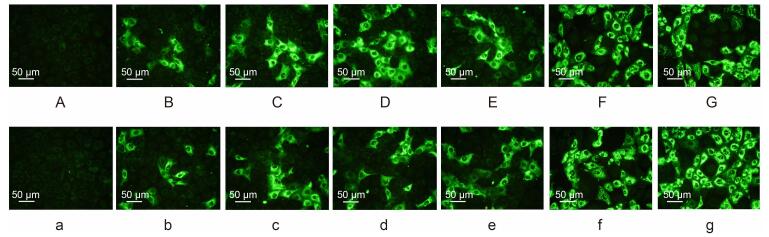
Figure 4. Immunofluorescence analysis of RV antigens in MA104 cells infected with SA11(A-G) and Wa (a-g) with antibodies against the vector protein VP6F (B/b); chimeric proteins 6F/G1[142](C/c), 6F/G2[87](D/e), and 6F/G4[208] (E/e); RV SA11(F/f); RV Wa (G/g); and antiserum derived from the guinea pig mock inoculated with PBS (A/a).(Bar:50 μm)
-
Antibodies in the serum of guinea pigs inoculated with chimeric proteins neutralized the infectivity of Wa and SA11 in MA104 cells(Table 1). Antibodies targeting the vector protein VP6F exhibited virus-neutralizing activity at lower titers. The neutralizing titers(1:640–1:2, 560)of antibodies from guinea pigs inoculated the chimeric proteins were significantly higher than these from VP6F-inoculated guinea pigs. The neutralizing titers in antiserum from negative control guinea pigs and in pre-immune sera from guinea pigs were less than 1:4.

Table 1. Neutralization activity of antibodies from chimeric proteins carrying the VP7 epitope inoculated guinea pigs.
Chimeric protein expression and purification
Antigenicity of the chimeric proteins
Immunofluorescence analysis of RV antigen
Neutralizing antibody activity
-
The group antigen protein VP6 is highly conserved, abundant, highly immunogenic, and antigenic. Although VP6 contains cross-reactive epitopes shared by other RVAs, suggesting that VP6-based vaccines could potentially provide heterotypic protection, the native VP6 lacks neutralizing antigenic contents that exist on the major antigenic proteins VP4 and VP7. Therefore, in this study, we evaluated the immunogenicities of chimeric VP6 proteins harboring three neutralizing epitopes derived from VP7 of RVA. Our data indicated that these antigens could elicit immunogenic responses and provide important insights into the development of novel vaccines.
Despite the significant reduction in RV-associated hospitalizations in industrialized and resource-deprived countries in recent years, the cost effectiveness of vaccination is affected in some countries by the high prices of the vaccines. Furthermore, the lower immunogenicity, reduced efficacy(Cunliffe et al., 2012; Patel et al., 2009; Sow et al., 2012; Zaman et al., 2010), and potential adverse effects of these vaccines are also problematic in developing countries(Anderson, 2008; Eng et al., 2012; Gilliland et al., 2012; Kollaritsch et al., 2015; Patel et al., 2010; Soares-Weiser et al., 2012; Studer et al., 2002; Yen et al., 2010). Therefore, the development of alternative vaccines is necessary in order to improve the efficacy and safety of live oral RV vaccines.
VP7 is the major antigen protein of RVAs. Antibodies against VP7 neutralized the infectivity of RVAs. In previous studies, neutralizing monoclonal antibodies and sequence analysis were utilized to locate amino acid residues found in antigenic positions on VP7 form selected RVAs; some specific epitopes were defined(Kirkwood et al., 1993). For example, amino acid residues 88–100, 142–152, 209–223, and 235–242 are involved in cross-reactive neutralization(Hoshino et al., 1994), whereas residues 5–13, 8–16, and 31–40 are RV-specific cytotoxic T lymphocytes(CTL)epitopes(Buesa et al., 1999). In this study, we selected three epitopes(G1[142], G2[87], and G4[208])derived from VP7 of RVA to develop chimeric vaccines in a foreign epitope presenting system with VP6 as the vector. Chimeric proteins were successfully expressed, and neutralizing activities of antibodies against these chimeric proteins were significantly higher than those against the vector protein VP6F. Moreover, the neutralization titers of anti-VP7 epitope chimeric proteins were as high as those of anti-VP4 epitope chimeric proteins described in a previous report(Teng et al., 2014), and the neutralization titers of the anti-vector protein(VP6F)antibodies were lower than those of anti-chimeric proteins and anti-Wa or anti-SA11 viruses. However, the antibodies showed obvious neutralizing activity, inhibiting replication in MA104 cells, similar to the results of a previous study, Therefore, these data supported that anti-VP6 antibodies possessed neutralizing activity in vitro.
Together with our previous study demonstrating the good immunogenicity of VP4 epitope chimeric vaccines using VP6 as a vector, we expect that a chimeric vaccine using VP6 as the vector and carrying a single VP4 or VP7 epitope will provide better protection against RV than a vaccine harboring only the VP6 protein. Additionally, a chimeric vaccine using VP6 as the vector and simultaneously carrying both VP4 and VP7 epitopes may provide another promising alternative to enhance immunization against RVAs.
However, while we have made great progress toward development of a chimeric vaccine using VP6 of RV as a vector, there are many challenges ahead. First, it will be essential for a recombinant chimeric vaccine to carry epitopes derived from the VP7 and VP4 in VP6 as a vector, simultaneously, because the major neutralizing epitopes of RVA exist mainly on VP7, and the major cross-neutralizing epitopes exist mainly on VP4. Second, VP6 can self-assemble into VLPs, and VLPs can improve antigen immunogenicity. Therefore, it will be interesting to determine whether VP6 maintains self-assembly ability after introduction of VP7 and VP4 epitopes. Third, the neutralization efficiency of sera derived from recombinant VP6s containing VP7 and VP4 epitopes will be lower than that derived from SA11 and Wa, because the whole virion of SA11 or Wa contains epitopes of other antigen proteins besides VP7 and VP4. Thus, it will be essential to compare the protection efficacies of new vaccines with currently available commercial RV vaccines.
-
This work was supported in part by the Foundation of the Applied Basic Research Project of Yunnan Province, 2013FZ130, and the Foundation of the Applied Basic Research Project of Yunnan Province Youth Program, 2012FD039.
-
The authors declare that they have no conflict of interest. All the animal tests comply with national and institutional guidelines established by the Institute of Medical Biology Animal Care and Use Ethic Committee, and the experiments were conducted under the guidelines of the Institute of Medical Biology Animal Care and Use Ethic Committee.
-
BZ, XP, YT, WX, JW and YW have participated plasmids construction, carried out protein expression, animal experiments and immunological analysis. YC has designed the study, supervised the data evaluation, and wrote the manuscript.















 DownLoad:
DownLoad: