-
Dear Editor,
Here, we report what we believe is the first case of EBVassociated HMB from Europe. Follow-up schedules and the necessity of reporting of EBV-associated HMB cases are being discussed. The median age at diagnosis of HMB is 6.7 years, and local cutaneous reactions include erythema, bullae, necrosis, and ulceration. In addition, systemic symptoms, including high-grade fever, malaise, lymphadenopathy, hepatosplenomegaly, hepatic dysfunction, hematuria, and proteinuria, are often present. While the etiology of HMB remains unclear, mosquito salivary gland extract may trigger EBV reactivation in latently infected NK cells. Upon reactivation, EBV oncogenes, such as latent membrane protein 1 (LMP1), may induce immortalization of NK cells, eventually progressing to lymphoma. One-third of patients with CAEBV will present with HMB, while the vast majority of HMB cases are attributed to EBV reactivation or CAEBV (Hall et al., 2015; Park and Ko, 2014; Tokura et al., 2001). Here, we report what we believe is the first case of EBVassociated HMB from Europe. Follow-up schedules and the necessity of reporting of EBV-associated HMB cases are being discussed.
We hereby describe the case of a 6-year-old native Greek boy with primary EBV infection who was ini-tiallyadmitted to our hospital with fever, cervicallymph-adenopathy, liver dysfunction, and splenomegaly. EBV viral capsid antigen (VCA) IgM was positive at 2.28 S/CO via CMIA Architect assay (negative reference val-ues < 0.5), while therespective IgG titer was negative at 0.17 S/CO (negative reference values < 0.75). The child soon improved regardingthe infectious mononucleosis syndrome (IMS), and 22 days later, his serologies were indicativeof past infection (EBV VCA IgM 0.87 S/CO, EBV VCA IgG 9.2 S/CO).
Two months after the initial admission, the child presentedwith erythema, bullae, and ulcerations in both upper and lower limbsand periorally. A detailed history revealed only mosquito bites (Figure 1A). Two days later, the child deteriorated under conservative treatment and developed fever, lymphadenopathy, and hepato-splenomegaly, along with further expansion of his skin lesions.Serological investigations demonstrated positive EBV VCA IgM of 1.36 S/CO and positive EBV VCA IgG of 21.9 S/CO, suggesting persistent EBV infection. Both IgM and IgG were negative for cytomegalovirus, Toxoplasma, herpes simplexviruses (HSV-1, HSV-2), Coxsackie A viruses, and parvovirus B19 in serological assays, which revealedneither recent nor past infection of those pathogens. Serum IgE was 38 IU/mL and #PLT was 20 × 104/μL, while liver enzymes were elevated (SGOT 61 U/L, SGPT 129 U/L, γ-GT 63 U/L). Figure 1B shows the patient's leg five days after the bullae erup-tion, while Figure 1C shows the scar lesion in the same leg six months after HMB incidence. Immunophenotyping was conducted in the peripheral blood in order to define lymphocyte subpopulations at the onset of HMB, as well as at four and six months after HMB onset (Table 1). Interestingly, the results revealed low B (CD19+) and T cell counts (CD3+) with especially low levels of T-helper (Th) cells (CD3+/CD4+) and a reversed helper-to-suppressor ratio in the acute phase of HMB. These findings do not support NK-mediatedEBV reactivation, a theory that hasbeen documented for previously reported cases of pediatricHMB. White blood cell (WBC) counts re-mained low four months later, with comparatively lower Th cells, although the T4/T8 ratio appeared normal. Serol-ogical assays for EBV VCA in the latter follow-up were marginally positive for IgM (0.77 S/CO) and positive for IgG (22.76 S/CO); along with a negative PCR for EBV in two consecutive blood samples, these indicated an in-fection in remission.Six months after HMB, EBV VCA IgM was negative (0.33 S/CO), while EBV VCA IgG was at a similarlevel (26.41 S/CO) and lymphocyte sub-sets were restored to normal. Follow-up investigations were conducted every two months for the first six months after HMB incidence and every six months thereafter for the next year, given that the patient's immunophenotype had normalized.
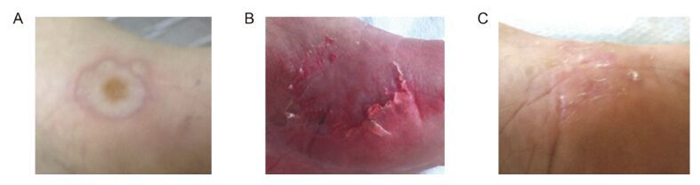
Figure 1. (A) Skin lesions during the initial HMB presentation; (B) Skin lesions in the same foot five days after bullae eruption; (C) Scar lesionssix months afterHMB incidence.
Marker Onset of HMB 4 months after HMB
(6 months after IMS)6 months after HMB
(8 months after IMS)Normal values for age % of lymphocytes Absolute values % of lymphocytes Absolute values % of lymphocytes Absolute values CD2+ 82.6 1346 ND ND 76 1854 - CD3+ (T) 80 1304 63 756 72 1757 1200-2600 CD3+/CD4+ (T)h 27.7 452 ↓ 30 360 ↓ 32 781 650-1500 CD3+/CD8+ (T)c 45.3 738 26 312 ↓ 35 854 370-1100 T4/T8 ratio 0.61 ↓ 1.15 0.9 CD19+ (B) 13 212 ↓ 21 252 ↓ 17 415 270-860 CD3-/CD (16+56)+ (NK) 8.9 145 13 156 8.5 207 100-400 CD3+/CD (16+56)+(NK-like T) 9.5 155 6 72 5.7 139 - TCR αβ+ 70 1141 52 624 ND ND - TCR γδ+ 9.5 155 9 108 ND ND - CD3+/CD4+/CD45RO+(Memory Th) 11.6 189 ND ND 9.6 234 230-630 CD3+/CD4-/CD45RO+(Memory Tc) 22.2 362 ND ND 12.4 303 70-390 CD3+/CD4+/CD45RA+(Naïve Th) 13.6 222 ND ND 22 537 320-1000 CD3+/CD4-/CD45RA+(Naïve Tc) 24.8 404 ND ND 30 732 310-900 CD3+/CD45RO+(Pan memory T) 33.6 548 ND ND ND ND - CD3+/CD45RA+(Naïve T) 38.4 626 ND ND ND ND - CD24+ ND ND ND ND 5 122 - CD7+ ND ND ND ND 80 1952 - HLA-DR+ ND ND ND ND 18 439 - CBC data WBC 4350/μL (#lym 1630/μL) WBC 4470/μL (#lym 1200/μL) WBC 5730/μL (#lym 2440/μL) - Note: ↓ = low value; CD = clusterof differentiation; Tc = cytotoxic T lymphocyte; TCR = T-cellreceptor; HLA-DR = Human leukocyteantigen -antigenD-related; CBC = completeblood count; #lym = lymphocytes count; ND = not determined; WBC = white blood count. Table 1. Lymphocyte subpopulations after development of HMB and during follow-up
HMB has beenreported to belinked to CAEBVand NK/T-cell leukemia/lymphoma. It has also been ob-served in non-EBV-related lymphoproliferative diseases, including chronic lymphocytic leukemia and mantle-cell lymphoma. Similarly, EBV-positive Hodgkin'slym-phoma has been reportedin a patient with HMB (Asada, 2007). According to an earlier review, HMB was classi-fied as a primary clinical manifestation of EBV-associated NK cell leukemia/lymphoma (Tokura et al., 2001). In this review, 90% of patients with HMB were found with NK lymphocytosis (range, 33.5%-67.3%), while NK cells in most of these cases contained monoclonal or occasionally biclonal EBV. Notably, half of the HMB patients died of hemophagocytic lymphohistiocytosis (HLH), granular lymphocyte proliferative disorder (GLPD), or lymphomas, and CAEBV was implicated in most of these cases.
A number of inconsistent reports have strengthened the perception that there is a clinical pattern involving(ⅰ)HMB, (ⅱ)EBV reactivation and/or CAEBV, (ⅲ)NK lymphocytosis, and(ⅳ)lymphoproliferative disorder. Apart from the 58 cases listed by Tokura et al.(2001), 34 additional cases of HMB that fulfilled at least three of these four clinico-laboratory criteria were identified via PubMed(as of September 12, 2016). In a case series of four young male Taiwanese patients with HMB and CAEBV, all four patients presented with EBV-infected NK cells, NK lymphocytosis(but not in all HMB episodes), and increased memory CD4+ levels along with high serum IgE levels. Furthermore, two of the four exhibited elevated activated lymphocytes(CD2+HLADR+), three had low CD4+ cell levels, two had low CD8+ cell levels, and one had low CD19+ cell levels(Lee et al., 2013). The last three findings were also present in our patient. Consistent with our case, low CD3+ and CD4+(± CD8+)counts were also documented in two of these studies(Chung et al., 2003; Roh et al., 2010).
There is one case in the literature referring to a 6-year-old Korean boy with HMB, EBV reactivation, and ab-sence of NK lymphocytosis (Seon et al., 2013). The ab-sence of NK lymphocytosis was also recorded in a 55-year-old woman who suffered from HMB and reactiva-tion of chronic EBV infection three years after begin-ning treatmentfor mantle-cell lymphoma. In this case, in situ hybridization revealed no EBV-encoded small nuclear RNA (EBER)-positive cells in skin lesions (Konuma et al., 2005). Several cases with NK lymphocytosis were also negativefor EBER (Roh et al., 2010). Interestingly, a recent case report of a 6-year-old girl from Taiwan closely resembled our case (Chiu et al, 2016), suggesting that HMB withoutNK lymphocytosis but with EBER-positive skin tissue may be the primary clinical manifesta-tion of an EBV infectionitself. This demonstrates that HMB can presentwith or withoutNK lymphocytosis and that EBV reactivation or CAEBV cannot be assumed. Apparently, EBV activity remains the key to the deve-lopment of HMB, but it is still unclear if caseswithout NK lymphocytosis should also be closely monitored in the contextof development of malignancy.
The mechanism by which mosquito antigen-specific reactions induce EBV reactivation remains to be elucidated. Immunohistochemical studies at the bite sites showed infiltration predominantly by CD4+ T cells and secondarily by CD8+ and CD16+ cells. Likewise, in situ hybridization demonstrated that 3%–10% of involved lymphocytes were EBV-positive, of which the vast majority were CD4+, suggesting a unique role for Th cells in the pathogenesis of HMB(Asada, 2007). In response to mosquito salivary gland extracts(SGEs), especially those of Aedes albopictus, CD4+ T cells proliferate and produce IL-4, a cytokine that induces differentiation of naïve Th cells to Th2 cells and is associated with allergic reactions. Notably, mosquito bites can induce expression of the EBV oncogene LMP1 in NK cells via antigen-specific CD4+ T cells and can activate basophils and/or mast cells, resulting in the development of the severe skin reactions seen in HMB(Asada et al., 2005; Sakakibara et al., 2015). In addition, a child with HMB associated with EBV infection and NK lymphocytosis showed a positive response to Culex pipiens, a species prevalent worldwide, after a skin-patch test(Roh et al., 2010). A. albopictus has been present in several Greek districts since 2008(Giatropoulos et al., 2012). Interestingly, a history of allergic reactions to insect bites alone is associated(OR 5.1; 95% CI: 1.4–19.2) with the development of immunoblastic non-Hodgkin's lymphoma(Briggs et al., 2002).
CD4+ T cells activated by mosquito SGEs lead to reactivation of latent EBV infection in NK cells, while EBV-specific CD4+ cells seem to play a potential role in the reactivation of latent EBV infection in resting B cells through a CD40-dependent pathway. In HMB patients, EBV-carrying NK cells overexpress surface or soluble Fas ligand(Fas L), an enhancement that is related to organ and tissue damage, such as intense skin reactions and liver dysfunction. Moreover, mosquito SGEs have been found to induce the expression of the BZLF1 gene(a viral lytic-cycle transactivator)at the bite site, suggesting EBV reactivation at these sites. Most patients with HMB have high titers of serum antibodies against EBV lytic-cycle proteins, including VCA and early antigen(EA), while increased plasma EBV copy numbers are common among the most severe cases. Beyond local skin reactions, cellfree EBV or EBV-infected B cells subsequently induce immune reactions that resemble those of IMS. EBV infection of B cells, in turn, induces the expression of superantigens by the host, leading to further T-cell activation(Asada, 2007; Tokura et al., 2005).
As mentioned above, the first step in HMB-associated EBV-positive NK lymphocytosis linked to the development of malignancies and NK proliferation seems to be the stimulation of CD4+ T cells by SGEs. While HMB may be the first manifestation of clonal EBV+ NK-cell malignancy, it may instead indicate other lymphoproliferative disorders(e.g., chronic lymphocytic leukemia, mantle-cell lymphoma, Hodgkin's lymphoma, anaplastic lymphoma, or kinase-positive anaplastic large cell lymphoma)or CAEBV. Recent reviews suggest confirmation via identification of EBV-infected lymphocytes in skin biopsies and regular clinical follow-ups in order to monitor for the development of lymphoproliferative disease(Hall et al., 2015; Park and Ko, 2014). The latest field data show that an age of onset > 9 years and the presence of BZLF1 mRNA in skin lesions are significantly associated with mortality. On the other hand, sex, specific clinical symptoms, liver enzymes, EBV DNA load, anti-EBV antibody titers(VCA, EA, and EBNA), EBER in situ score, and type of lymphocyte subset involved(either NK or T)do not appear to be associated with mortality. Not surprisingly, low platelet count and splenomegaly failed to correlate with poor prognosis(Miyake et al., 2015). Thus, identification of BZLF1 mRNA in HMB cases seems to be one of the best choices for predicting the outcome, while interleukin-2(IL-2) levels may be helpful in predicting NK cells involvement(Suzuki et al., 2010).
In conclusion, HMB should not be ignored, even during the course of primary EBV infection. Independent of EBV infection status, the strong linkage of HMB with lymphoproliferative disorders indicates a need for high clinical monitoring and regular follow-up.
HTML
-
The authors declare that they have noconflict of interest. Written consent was obtained from the children's parents involved in the study.















 DownLoad:
DownLoad: