HTML
-
Exosomes are nanovesicles ranging in size from 30 to 150 nm that are released from all cells, allowing them to be detected in various body fluids (Simons and Raposo 2009; Thery et al. 2002; Yanez-Mo et al. 2015). Exosomes were originally discovered as secreted waste products in studies of the transformation of reticulocytes into mature red blood cells. However, exosomes have recently been described as intraluminal vesicles (ILVs), which form multivesicular bodies (MVBs) that subsequently fuse with the cytoplasmic membrane to be released into the extracellular space (Harding et al. 2013; Thery et al. 2002). Exosomes not only contain waste discarded by cells but also play important roles in disease and pathology, particularly infectious diseases (Anderson et al. 2016; Chahar et al. 2015; Liu et al. 2017; Meckes and Raab-Traub 2011; Nagashima et al. 2014).
Rabies virus has a negative single-stranded RNA genome, and in some animals who are seropositive for rabies, the viral RNA genome is detectable using polymerase chain reaction (PCR) on saliva samples, but no virus particles can be directly detected (Warrell and Warrell 2004). Additionally, it is still unclear how rabies virus spreads among host cells (Schnell et al. 2010). Exosomes can have opposing roles in viral infections. First, infected cells shed exosomes containing viral proteins and the whole virus genome, promoting the spread of infection to other cells (Nour and Modis 2014; Raab-Traub and Dittmer 2017; Simons and Raposo 2009). This mechanism of virus spreading can potentially help the virus evade host immune defenses, such as neutralizing antibodies. However, exosomes can also transfer antiviral molecules induced by host immune responses to inhibit virus replication (Raab-Traub and Dittmer 2017; Schwab et al. 2015). For example, human immunodeficiency virus (HIV)-1 Nef protein presented in exosomes shed from virus-infected cells can induce apoptosis of CD4+ T cells (Luo et al. 2015). Thus, the pathogenicity of HIV-1 is achieved by eliminating the action of CD4+ T cells (Fevrier et al. 2011). Moreover, Nef protein can be transmitted from infected macrophages to uninfected B cells through the vesicle transport pathway (Lenassi et al. 2010). Exosomes from HIV-infected cells contain transactivating response (TAR) element RNA and can stimulate the expression of pro-inflammatory cytokines in primary macrophages (Sampey et al. 2016). Viral proteins and whole-virus genomes were found to be present in exosomes secreted by hepatitis C virus (HCV)-infected cells, enabling the establishment of infections and resistance to neutralizing antibodies (Liu et al. 2014; Ramakrishnaiah et al. 2013). Exosomes derived from human T-lymphotropic virus type 1 (HTLV-1)-infected cells incorporated viral proteins and mRNA and played important roles in HTLV-1-induced pathogenesis (Jaworski et al. 2014). Enterovirus 71 (EV71)-infected cells secrete exosome-packaged viral genomic RNA and miR-146a, facilitating viral pathogenesis (Fu et al. 2017). Exosomes secreted from Epstein-Barr virus-infected B cells were also found to harbor virus-associated microRNA (miRNA), and Epstein-Barr virus-infected nasopharyngeal carcinoma (NPC) cells secrete exosomes with viral miRNAs that can be transported into uninfected cells (Baglio et al. 2016; Meckes et al. 2010; Verweij et al. 2013). In hepatitis B virus (HBV)-infected cells, interferon-induced molecules are transferred intercellularly via exosomes to serve antiviral proteins (Li et al. 2013).
Although there are many studies focus on the roles of exosomes in virus infection process, the exact function and detailed mechanism of exosomes during the process need further investigation. How the rabies virus spreads among host cells and the role of exosomes in rabies virus infection process still remain unclear. In this study, we explored the roles of exosomes in rabies virus infection using OptiPrepTM density gradient centrifugation to isolate exosomes from rabies virus-infected Vero cell culture supernatants and demonstrated that rabies virus infection enhanced the production of exosomes, which may facilitate virus infection. Exosomes may facilitate infection and the spread of viruses. Our results provide important insights into the mechanisms by which exosomes facilitate the spread of viruses in host cells.
-
Vero cells were cultured as monolayers in Dulbecco's modified Eagle's medium supplemented with 10% exosome-free fetal calf serum (depleted of bovine exosomes by ultracentrifugation for 16 h at 100, 000 ×g), 100 IU/mL penicillin, and 100 mg/mL streptomycin at 37 ℃ in an atmosphere containing 5% CO2. Vero cells were infected with rabies virus (PM1503 strain) at a multiplicity of infection (MOI) of 0.01, and the infected cells were cultured for 4 days before exosome isolation.
-
Transmission electron microscopy (TEM) was used to observe the morphological characteristics of the exosomes. The Vero cells (approximately 2 × 106) were fixed with 4% glutaraldehyde and osmic acid at 4 ℃ followed by gradient elution using ethanol. Cells were then embedded for 3 h, sectioned using an ultramicrotome (LEICA EM UC7), and stained with uranyl acetate and lead citrate. The purified exosomes were negatively stained using 2% (w/v) phosphotungstic acid and were immunolabeled with antiCD63 antibodies. The imaging was performed at an acceleration voltage of 80 kV using a transmission electron microscope (HITACHI H-7650).
-
Exosomes were isolated using differential velocity centrifugation followed by OptiPrepTM density gradient centrifugation. Supernatants harvested from Vero cells (approximately 4 × 108) were centrifuged at 4 ℃ and 300 ×g for 10 min, 2000 ×g for 20 min, and 10, 000 ×g for 30 min (Théry et al. 2006). Pellets containing cells, dead cells, and cell debris from each of the first three centrifugations were discarded, and the supernatants were used in the next step. After centrifugation at 100, 000 ×g for 2 h at 4 ℃, the supernatants were discarded, and the pellets (containing exosomes and rabies virus particles) were resuspended in 500 μL phosphatebuffered saline (PBS). Subsequently, an OptiPrepTM density gradient was used to purify the exosomes from rabies virus, as previously described (Cantin et al. 2008; Konadu et al. 2016). A discontinuous 6%–24% density gradient was prepared by diluting solutions of 24%, 22%, 20%, 18%, 16%, 14%, 12%, 10%, 8%, and 6% (w/v) iodixanol from a stock solution of OptiPrepTM (60% [w/v] aqueous iodixanol [Axis-Shield PoC AS, Oslo, Norway]) with 0.25 mol/L sucrose/TE buffer (pH 7.4). The unpurified exosomes (containing rabies virus particles) in a volume of 500 μL were overlaid on the top of the gradient and centrifuged using a Beckman Coulter SW40Ti rotor at 100, 000 ×g for 18 h at 4 ℃.
-
After centrifugation, each gradient fraction was collected manually. The exosomes and rabies virus particles in the fractions were identified by acetylcholinesterase (AChE) assay and rabies virus G protein enzyme-linked immunosorbent assay (ELISA), respectively. These two assays were performed according to the product instructions. AChE is an exosome-specific marker that has been investigated in previous studies (Cantin et al. 2008; Gastpar et al. 2005; Konadu et al. 2016; Rieu et al. 2000).
-
The sizes and concentrations of the purified exosome fractions were measured using a ZetaView NTA (Particle Metrix GmbH, Meerbusch, Germany), which is a combination of light scattering and Brownian motion technology. The assays were performed according to the user guide of the NTA. Briefly, the exosome sample was diluted in 1 mL PBS and loaded into the cell. The instrument measured each sample at 11 different positions throughout the cell. For each measurement, the instrument pre-acquisition parameters were set to a temperature of 25 ℃, a sensitivity of 85, a frame rate of 30 frames per second (fps), a shutter speed of 100, and a laser pulse duration equal to that of shutter duration.
-
Exosome fractions (8%–12%) were pooled, diluted with 3 mL PBS, and ultracentrifuged at 100, 000 ×g for 2 h. After discarding the supernatant, the pellets were resuspended in radioimmunoprecipitation assay (RIPA) buffer and heated for 10 min at 97 ℃. The denatured proteins were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 12% gels and transferred onto nitrocellulose membranes using a Bio-Rad dry blotting system (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 3% nonfat powdered milk in PBS for 1 h at 20–25 ℃ and then incubated with primary anti-cluster of differentiation 63 (CD63) antibodies (1:1000, System Biosciences, Palo Alto USA), anti-CD81 antibodies (1:1000, Abcam, Cambridge, UK), anti-TSG101 antibodies (1:1000, Abcam), and anti-calnexin antibodies (1:1000, Abcam) overnight at 4 ℃. The membranes were washed three times with PBS plus 0.1% Tween (PBST) and then incubated with alkaline phosphatase-conjugated secondary antibodies (1:2000) for 1 h.
-
Vero cells (approximately 4 × 108) were treated with GW4869, or transfected with small interfering RNA (siRNA) against Rab27a (si-Rab27a), or controls. After 48 h, exosomes were isolated by using differential velocity centrifugation followed by OptiPrepTM density gradient centrifugation.
-
The MiniBEST viral RNA extraction kit (TaKaRa, Shiga, Japan) was used to extract rabies viral RNA. A One-Step SYBR PrimeScript RT-PCR kit (TaKaRa) and rabies virusspecific primers for the N gene (forward primer: 5'-CAAGATGTGTGCYAAYTGGAG-3' and reverse primer: 5'-AGCCCTGGTTCGAACATTCT-3') were used for amplification and subsequent quantification with a CFX96 system (Bio-Rad).
-
Data represent at least three independent experiments were presented as mean ± SEM and analyzed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). The Student's t test or one-way analysis of variance was used for statistical analysis to compare the differences among treatment groups. P < 0.05 was considered to indicate statistical significance.
Cell Culture and Virus
Transmission Electron Microscopy (TEM)
Isolation of Exosomes Using OptiPrepTM Density Gradient Centrifugation
Identification of OptiPrepTM Density Gradient Centrifugation Fractions
Nanoparticle Tracking Analysis (NTA)
Western Blot Analysis of Isolated Exosomes
Exosome Secretion Inhibition Assay
RNA Extraction and Reverse Transcription (RT)-PCR Analysis
Statistical Analysis
-
Exosomes are essentially ILVs generated by inward budding of endosomal MVBs. The ILVs of MVBs may traffic to the plasma membrane, where they are released into the extracellular space by fusion with the plasma membrane (Harding et al. 2013; Simons and Raposo 2009). Accordingly, we investigated the release of exosomes from rabies virus-infected Vero cells using ultrathin sections fixed with 2% glutaric dialdehyde. As shown in Fig. 1A, MVBs invaginated inward within the cell. Additionally, ILVs were fused with the plasma membrane and released the exosomes into the extracellular environment (Fig. 1B).

Figure 1. Transmission electron microscopy (TEM) images of exosomes in rabies virusinfected Vero cell ultrathin sections. A The arrow indicates ILVs that formed from MVBs invaginated inward. B Formed ILVs were fused with plasma membrane. ILVs: intraluminal vesicles; MVB: multivesicular body; PM: plasma membrane. Scale bars = 500 nm.
-
One major challenge in this study was that exosomes and rabies viruses have similar buoyant densities. Therefore, a simple and reliable method was needed for isolating and purifying exosomes from rabies virus-infected cell culture supernatants. In this study, we used a 6%–24% OptiPrepTM density gradient centrifugation method to isolate exosomes from rabies viral particles. The centrifugation fractions were identified using AChE assays and rabies virus G protein ELISA. As shown in Fig. 2, the exosomes and rabies viral particles were present in the 8%–12% and 18%–20% fractions, respectively.

Figure 2. OptiPrepTM density gradient centrifugation technique to separate exosomes from rabies virus-infected cell supernatants. Fractions from 6%–24% OptiPrepTM density gradient were collected and analyzed using AChE activity assays (A) and rabies virus G protein ELISA (B). All data are means of three independent experiments; AChE, acetylcholinesterase; ELISA, enzymelinked immunosorbent assay.
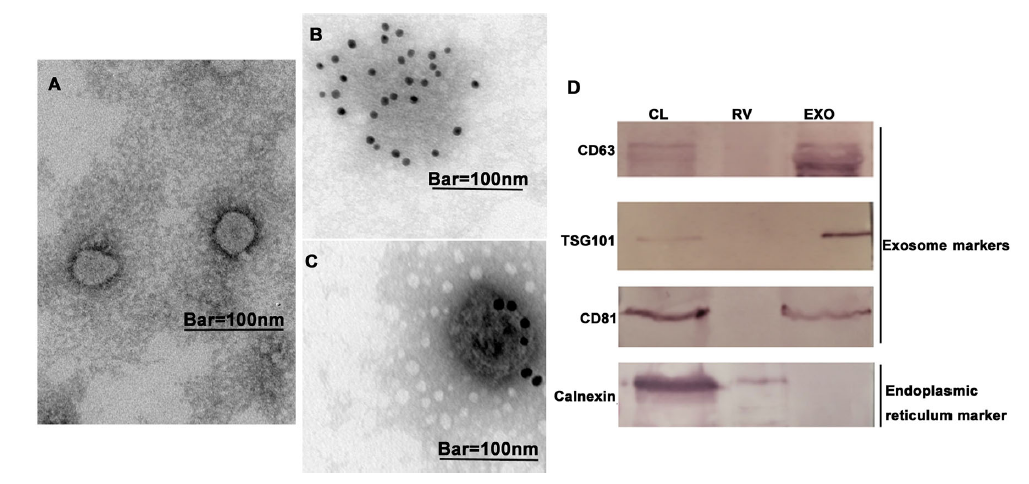
Next, we further characterized the isolated exosomes using TEM and western blotting. As shown in Fig. 3A-C, the exosomes were negatively stained with phosphotungstic acid and immunogold labeled with anti-CD63 antibodies. The TEM data showed that the exosomes had a round morphology. As shown in Fig. 3D, the exosome fractions (8%–12%) had positive signals of exosome-specific markers (CD63, CD81, and TSG101) and were negative for calnexin, a marker of the endoplasmic reticulum.

Figure 3. Characterization of exosomes from rabies virus-infected cell culture supernatants. Transmission electron microscopy (TEM) analysis shows purified exosomes negatively stained with (A) 2% phosphotungstic acid and (B, C) immunogold labeled with anti-CD63 antibodies. Scale bars = 100 nm. D Western blot analysis of purified exosomes using exosome-specific markers CD63, CD81, and TSG101 and nonexosomal marker calnexin (CL: cell lysate; RV: rabies virus fraction; EXO: exosome fraction). Results are representative of three independent experiments.
Taken together, these exosome-specific markers were found only in the exosome fractions (8%–12%), and the isolated exosomes had a round morphology. These data indicated that OptiPrepTM density gradient centrifugation could be used to isolate exosomes from rabies virus-infected cell culture supernatants. The data of the rabies virus G protein ELISA implied that the exosome fractions did not contain virus particles.
-
We then investigated the effect of virus infection on exosome secretion. The exosomes were isolated from the same volumes of virus-infected cell culture supernatants and control cell culture supernatants. As shown in Fig. 4A, the number of exosomes isolated from virus-infected cell culture supernatants (approximately 5.2 × 107/mL) was significantly (P < 0.05) higher than that in the control group (approximately 1.3 × 107/mL).
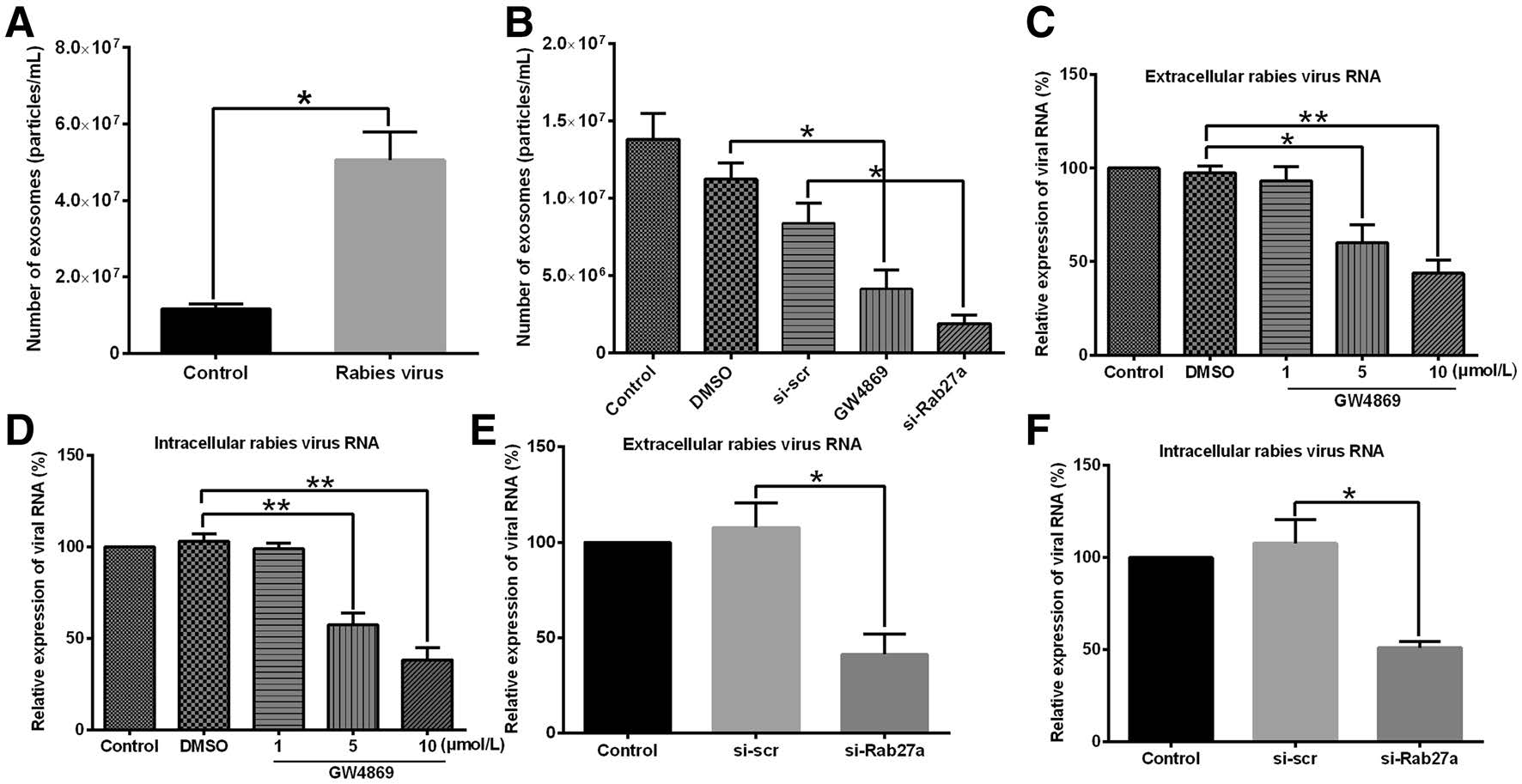
Figure 4. Quantification of exosomes released from Vero cells and effects of exosomal secretion inhibitors on rabies viral infection process. (A) Numbers of exosomes in uninfected Vero cell culture supernatants and rabies virus-infected Vero cell culture supernatants were measured using nanoparticle tracking analysis (NTA). (B) Exosomal secretion inhibitors GW4869 and si-Rab27a blocked exosome release. Exosome number was measured using NTA. (C, D) GW4869 reduced levels of extracellular and intracellular viral RNA. Vero cells were pretreated with 1, 5, and 10 μmol/L GW4869; DMSO; or culture medium (control) for 12 h before addition of rabies virus. Then, 48 h after infection, extracellular and intracellular viral RNA was extracted, and RT-PCR was used to quantify rabies viral RNA levels. Relative expression viral RNA levels were determined and standardized with 18S rRNA. (E, F) si-Rab27a reduced extracellular and intracellular viral RNA. Vero cells were transfected with small interfering RNA (siRNA) against Rab27a (si-Rab27a) or control siRNA (si-scr) for 12 h before infection with rabies virus. Next, rabies virus RNA was extracted from extracellular and intracellular fractions at 48 h after infection and quantified using RT-PCR. Relative expression viral RNA levels were determined and standardized with 18S rRNA. Relative expression of viral RNA in DMSO-, GW4869-, and si-Rab27atreated groups in (C–F) was calculated as percentage of viral RNA in control group, which was set at 100%. All data are from three independent experiments (*P < 0.05 and **P < 0.01).
Next, the roles of exosomes in the viral infection process were investigated. GW4869 and si-Rab27a, two exosomal secretion inhibitors, were used to reduce the release of exosomes. As shown in Fig. 4B, both GW4869 and si-Rab27a effectively inhibited exosome release. Then, Vero cells were pretreated with 1, 5, or 10 μmol/L GW4869 for 12 h before the addition of rabies virus. Then, 48 h after viral infection, the extracellular and intracellular rabies viral RNA was quantified using RT-PCR. As shown in Fig. 4C, 4D, treatment with GW4869 significantly reduced the levels of extracellular and intracellular rabies virus RNA. When cells were treated with 100 nmol/L si-Rab27a, we also found that the levels of intracellular and extracellular rabies viral RNA were significantly reduced by the inhibitor (Fig. 4E, 4F).
These results demonstrated that viral infection increased exosome secretion. Moreover, exosomal secretion inhibitors reduced the levels of extracellular and intracellular viral RNA. These data indicate that exosomes may participate in the process of rabies viral infection.
Observation of Exosomes in Ultrathin Cell Sections
Isolation and Identification of Exosomes
Exosome Pathway may Participate in Rabies Virus Infection
-
Exosomes are membrane-bound vesicles secreted from most cell types and found in various biological fluids (Alenquer and Amorim 2015; Simons and Raposo 2009). In this study, we used TEM to observe exosome release from rabies virusinfected cells. MVBs were clearly shown to invaginate inward, forming ILVs that fused with the plasma membrane to release the exosomes into the extracellular environment.
Several methods are currently used for the isolation and purification of exosomes from cell culture supernatants including differential centrifugation, sucrose density gradient centrifugation, ultrafiltration, and antibody-coated immunomagnetic using commercialized kits (Momen-Heravi et al. 2013). However, these commonly used techniques for isolating exosomes result in contamination with viral particles because exosomes and virus particles have similar sizes and densities. An OptiPrepTM density gradient centrifugation protocol for isolating exosomes from HIV particles was established in previous studies (Cantin et al. 2008; Konadu et al. 2016). In the present study, considering the characteristics of large high-density rabies virus particles versus relatively small low-density exosomes, we modified the 6%–18% OptiPrepTM density to 6%–24%. Our data demonstrated that the modified gradient centrifugation could be used to isolate exosomes from the supernatants of rabies virus-infected cells.
Several recent studies have confirmed that exosomes play important roles in physiological and pathological environments by carrying proteins, mRNAs, miRNAs, and other host functional genetic elements to neighboring cells, thereby facilitating the establishment of productive infections and modulating cellular responses. In this study, we found that the rabies viral infection increased the release of exosomes from host cells. Moreover, GW4869 and siRab27a treatments inhibited exosome production and reduced levels of extracellular and intracellular viral RNA. These findings are similar to those of Huang et al. who reported that Zika virus (ZIKV) infection in human fetal astrocytes induced a significant increase in extracellular vesicles and treatment with GW4869 decreased extracellular and intracellular viral RNA levels (Huang et al. 2018). Various viruses including HIV, HBV, HCV, rabies virus, herpes viruses, filoviruses, and arenaviruses need or hijack the endosomal sorting complexes required for transport (ESCRT) pathway for their release (Alenquer and Amorim 2015; Chahar et al. 2015; Liu et al. 2017; Nagashima et al. 2014; Schorey and Harding 2016), and the ESCRT pathway has been shown to play a role in the formation and secretion of exosomes. The reduction of intracellular viral RNA may be due to the reduction of extracellular virus. The number of progeny viruses in the extracellular medium was decreased, which may reduce the overall viral infectivity. Another possibility is that the function of exosomes in the viral infection process may not only contribute to virion release but also affect other processes in the viral life cycle. The detailed mechanisms mediating the reduction of intracellular viral RNA levels in exosomal secretion inhibitor-treated cells need to be investigated further.
Although we obtained some interesting findings, there are several certain limitations of this study. One is that which step of rabies virus infection process is regulated by exosomes. The other limitation is that the exact molecule(s) of exosomes and molecular mechanism involved in the regulation process of exosomes remains need to be revealed in future.
In conclusion, our data demonstrated that the 6%–24% OptiPrepTM density gradient centrifugation could be used to separate exosomes from rabies virus virions. Moreover, rabies virus infection enhanced the release of exosomes. GW4869 and si-Rab27a inhibited exosome release and reduced the levels of extracellular and intracellular viral RNA. These data indicate that exosomes may participate in the process of viral infection. Our results establish a basis for future research into the roles of exosomes in rabies virus infection and as potential targets for developing new antiviral strategies.
-
This work was supported by the National Natural Science Foundation of China (Grant No. 31770184) and Construction Project of Provincial-School of Jilin Province (No. 440050316A28).
-
JW, AH, and CJ contributed to the conception of the study. JW, AH, and FW contributed to designed and performed the experiments. CL, WD, and YT contributed assisted performed the experiments. JW, WS, FG, WK, LC, AH, and CJ contributed analysis with constructive discussions. CJ contributed to resources. JW, AH, and CJ contributed significantly to analysis results and manuscript preparation. JW, LC, AH, and CJ contributed review and editing manuscript. All authors read and approved the final manuscript.
Acknowledgements
Author Contributions
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.















 DownLoad:
DownLoad: