HTML
-
Dengue virus (DENV) is a mosquito-borne flavivirus (family Flaviviridae, genus Flavivirus) that is endemic throughout much of the tropical and subtropical regions (Pang et al. 2017). DENV has become the most serious mosquito-borne disease in southern China, particularly in Guangdong Province since it reemerged in Foshan City, Guangdong Province, in 1978 (Bai et al. 2018; Sun et al. 2017; Xia et al. 2018). All four serotypes of DENV have been associated with dengue fever, dengue haemorrhagic fever, and dengue shock syndrome in humans. The recurrent dengue epidemic in Guangdong Province has involved all four serotypes of DENV and DENV-1 has become the dominant serotype in the last decade (Bai et al. 2018; Sun et al. 2017; Xia et al. 2018). Phylogenetic studies of DENV from Guangzhou have suggested that DENV isolates that cause seasonal epidemics often have high sequence identity with DENV isolates reported in the previous year (Luo et al. 2017; Sang et al. 2015), and autochthonous infection cases in Guangzhou also have been recorded (Luo et al. 2017), which indicates that DENV may have established a transmission cycle that is endemic to humans in Guangzhou.
The urban transmission cycle of DENV is dependent on competent mosquito vectors and susceptible human populations intersecting within a permissive environment. Humans are the only primary vertebrate hosts in the urban cycle. Aedes aegypti (Ae. aegypti) and Ae. albopictus are universally acknowledged as the principal vectors of DENV. However, among the human population in Guangzhou, Ae. aegypti is not detected, and Ae. albopictus is the only important vector of DENV and is thus responsible for DENV transmission (Li et al. 2019; Luo et al. 2017; Zhang et al. 2019). There are no infection cases in Guangzhou, which has a humid subtropical climate influenced by the Asian monsoon (Lu et al. 2009), during the colder winter months. Most Ae. albopictus in Guangzhou are quiescent or diapause during cold winter months. It has been reported that Ae. albopictus mosquitoes could transmit DENV to their offspring in Guangzhou under laboratory conditions (Chen et al. 2019). Despite the widespread impact of DENV on human communities, the transmission dynamics of this virus have not been fully elucidated, particularly regarding how the virus persists in Guangzhou when cold temperatures obviate viral replication and decrease vector blood-feeding activity. To advance dengue control, it is necessary to elucidate the overwintering mechanisms of DENV, because they determine the prevalence of the virus at the termination of winter and thus may determine the force of viral transmission during vernal amplification.
Ae. albopictus distribution in winter (December– February) was limited to a subtropical-tropical area of China, and Ae. albopictus exhibited blood sucking activity (Li et al. 2002), primarily laid eggs in containers such as balcony flowerpots in Guangzhou in winter (Zhong et al. 2019) and fed primarily on rodents in the wilding urban areas (Goodman et al. 2018; Yang et al. 2020). In this study, a mock microenvironment urban balcony ecosystem containing fundamental elements of DENV transmission dynamics (DENV-positive Ae. albopictus, hydroponics flower (food), beakers containing water (breeding ground) and suckling mice (blood meal)) was established, and the profile of the mosquito population and DENV kinetics in mosquitoes under the simulated Guangzhou balcony environment were observed from October, 2016 to June, 2017. The results of this study may provide laboratory evidence for autochthonous cases of DENV among native-born Chinese individuals in Guangzhou with no history of foreign exposure.
-
C6/36 cells (ATCC CRL-1660) were maintained in 1640 medium with 10% fetal bovine serum (FBS) at 28 ℃ with 5% CO2. The DENV-1 used in this study was isolated from a patient in Guangzhou in 2014 (GZ2014001, Accession No: KM925072) and propagated in C6/36 cells as described previously (Chen et al. 2019). After cytopathic effects of viral infection were observed, the viral suspension was collected, titrated as described previously (Li et al. 2011) and stored at - 80 ℃ until use.
-
Ae. albopictus colonies were established and maintained as described previously (Chen et al. 2019). In brief, colonies were maintained at an indoor temperature (27 ℃–31 ℃), with 60%–85% relative humidity (RH), and a 12:12 light: dark cycle. The larvae were provided beef liver powder (Argentine, USA) and adult mosquitoes were maintained with 10% glucose solution and allowed to mate freely in cages that measured 30 cm × 30 cm × 40 cm. Those F2 mosquitoes were tested, as well as appropriate controls, by RT-PCR to confirm the absence of DENV, as described previously (Chen et al. 2019). F3 eggs were hatched, reared to adults and used in subsequent experiments.
-
One- to three-days-old Kunming suckling mice were purchased from the Central Animal Facility of the Southern Medical University and housed in specific pathogen free (SPF) conditions at the Center for Disease Control and Prevention of Guangzhou Military Command (GZMCCDC). All procedures were approved by the Animal Ethics Committee of the GZMCCDC (Protocol: 2015–104).
-
Kunming suckling mice were infected intracranially with DENV-1, and Ae. albopictus was fed with an infectious meal as described previously (Chen et al. 2019). Briefly, after inoculation, clinical signs of those suckling mice were recorded daily. Female 5- to 7-day-old mosquitoes were allowed to bite the abovementioned suckling mice when clinical signs of anorexia, limb paralysis, and lassitude were observed. The engorged mosquitoes were singled out in cages. All of the infected female mosquitoes fed DENV-1-infected blood meals via infected animals were randomly allocated into three cages at a mean of 100 mosquitoes per cage.
-
The study began on October 16, 2016 and ended on June 16, 2017. Three hundred female mosquitoes infected via infected animals were allocated to cages that had a 1-L beaker containing 800 mL of water and the hydroponic flower Epipremnum aureum. Those mosquitoes were maintained with the juice of Epipremnum aureum and randomly allowed to feed on a litter of 1–3 day-old Kunming suckling mice (9–13 suckling mice) for 2 h after infectious blood meals every 10 days. The blood of those suckling mice was collected for analysis at 5 days after the mosquito bites occurred, and viraemia was determined as described previously (Chen et al. 2019). The mosquitoes were sampled every month. The ambient temperature, humidity and number of mosquitoes at each observation time point were recorded.
-
The mosquitoes and larvae were tested separately for the presence of DENV-1 RNA as described previously (Chen et al. 2019). Briefly, larvae and whole mosquitoes were separately homogenized for 2–3 min with a Tissue Lyser (Qiagen, Valencia, CA) in 1.5-mL microcentrifuge tubes containing 0.3 mL 1640 medium with 2% FBS diluent on ice. Next, 200 μL aliquots were taken from each sample and dispensed into a 1.5-mL microcentrifuge tube containing 1 mL of TRIzol reagent (TaKaRa, Dalian, Liaoning Province, China) and total nucleic acids were extracted from each sample according to the manufacturer's instructions.
Mosquitoes and suckling mice were scored as DENVinfected using RT-qPCR assay on homogenized tissue, and the results were expressed as negative/positive (the threshold cycle number (Ct) was determined for each sample and a negative result indicating no RNA detection corresponded to any Ct value that was ≥ 40 cycles).
-
Viral RNA for the RT-qPCR assays was extracted and tested as described previously (Chen et al. 2019). Briefly, RNA was extracted from 200 μL aliquot and eluted in 50 μL of RNase-free ultrapure water. DENV in the samples was detected using a Quant one step RT-qPCR Kit (Probe) (Tiangen Company, Beijing, China) as previously described (Chen et al. 2019). RT-qPCR reactions were performed in 96-well reaction plates in a C1000TM Thermal Cycler machine (Bio-Rad, US). The detection system contained the following: 0.4 μL enzyme mix, 10 μL 29 reaction mix, 1 μL DENV-1-specific forward primer (10 μmol/L), 1 μL DENV-1-specific reverse primer (10 μmol/L), 0.4 μL fluorogenic probe(10 μmol/L)(primers designed according to the conserved sequence in DENV-1 NS5 protein: DV1-001R, 5′-GTGGAGAGGAACCTTGTG AAACC-30; DV1-001F, 5′-CTGTGACTTTCTTCAGCCC AGC-3′; DV1-001 Probe: 5′-6-FAM-TGACCAGCCACCT CTTCCCAACCGA-BHQ-1–3′), 2.2 μL H2O, and 5 μL sample. The PCR program was as follows: 54 ℃ for 30 min and 2 min at 95 ℃, followed by 40 cycles of PCR (15 s at 95 ℃ and 1 min at 60 ℃), and 1 s at 45 ℃. A negative control (water) and a positive control standard (the DENV-1 strain GZ2014001, RNA, 10–2 dilution) were included in each 96-well reaction plate.
-
IBM SPSS Statistics software (version 22) was utilized for statistical analysis. Differences in infectivity and engorgement between groups were analyzed using Student's t-test and the chi-square test. P-values of ≤ 0.05 were considered to indicate significant differences.
Viruses and Cells
Ae. Albopictus Mosquitoes
Experimental Animals
Experimental Exposure of Infected Animals to Ae. albopictus Mosquitoes
Simulation of Overwintering Ae. albopictus in Guangzhou
Determination of Infection Status
Reverse Transcription-Quantitative PCR (RT-qPCR)
Statistical analysis
-
Data collection was initiated on October 16, 2016, and ended on June 15, 2017. The temperature date for the study period were showed in Fig. 1A. The highest and lowest temperatures of the observation day were obtained from Weather China (http://www.weather.com.cn/weather/101280109.shtml). The ambient temperature and humidity of the experimental place were measured using a digital hygrometer (Kejian, China). During the study, the temperature in Guangzhou ranged from 10 ℃ to 34 ℃, the coldest temperature was observed on February 10 (10 ℃), the warmest was observed on June 5 (34 ℃), and the corresponding ambient temperatures were 11 ℃ and 29 ℃. The ambient humidity of the experimental location ranged from 50% to 97% and most humidity measurements were above 80%.
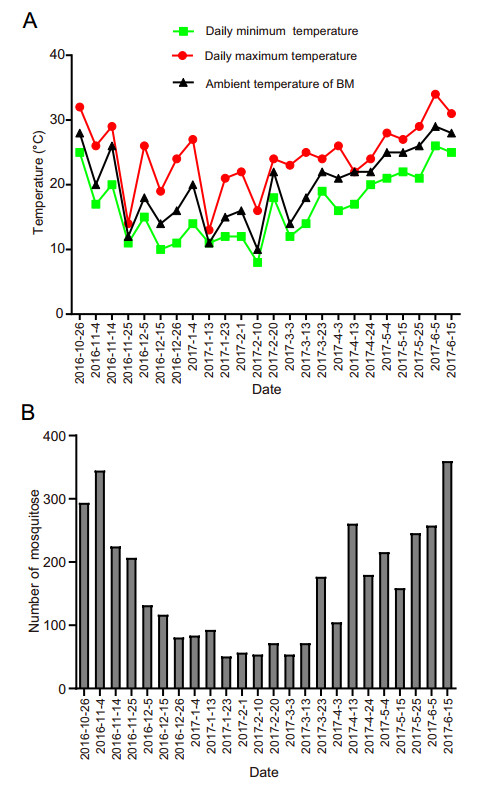
Figure 1. Temperature data and number of overwintering Aedes albopictus mosquitoes on the observation day. A Temperature data on the observation day. B Number of overwintering Ae. albopictus mosquitoes on the observation day.
Three hundred engorged female mosquitoes were randomlyallocated intothreecages atameanof100 mosquitoes per cage on October 16, 2016, after fed by DENV-1-infected blood meal via infected animals. Mosquito, larvae and pupae were observed throughout the experiment, and the numberof Ae. albopictus was monitored in our study. The mosquitoes exhibited oviposition activity 2 days after the first blood meal; the eggs hatched four days after the first blood meal; 3-or 4- star larvae were observed 13 days after the first blood meal; the pupae were grown 14 days after the first blood meal; and male mosquitoes emerged 15 days after the first blood meal via infected animals. The dynamics of Ae. albopictus in the study exhibited strong seasonal variability, as shown in Fig. 1B. The number of mosquitoes was maximal in early June, late May and October and was minimal in January and February. The results showed evidence of continuous adult, larval and pupal activity during winter in Guangzhou.
-
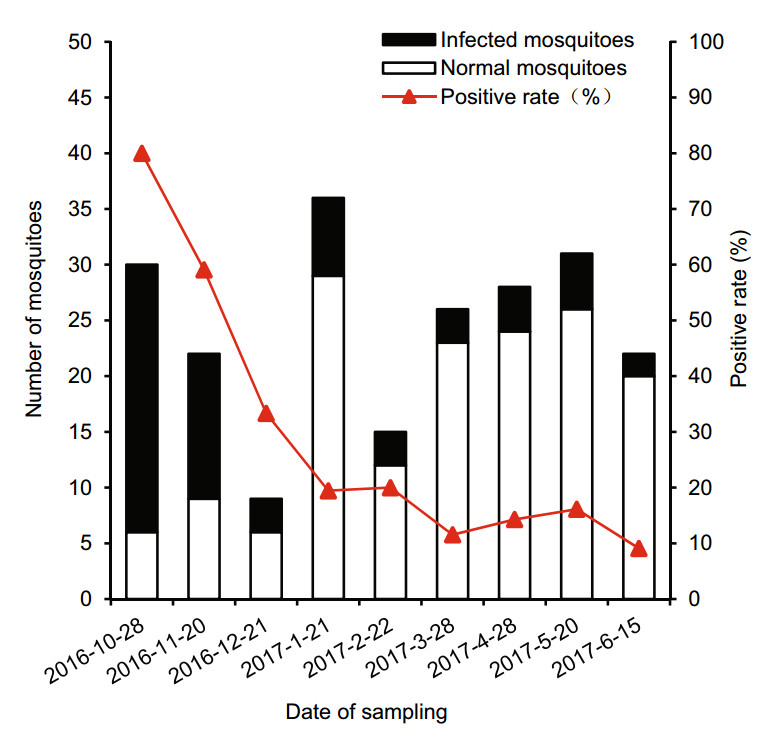
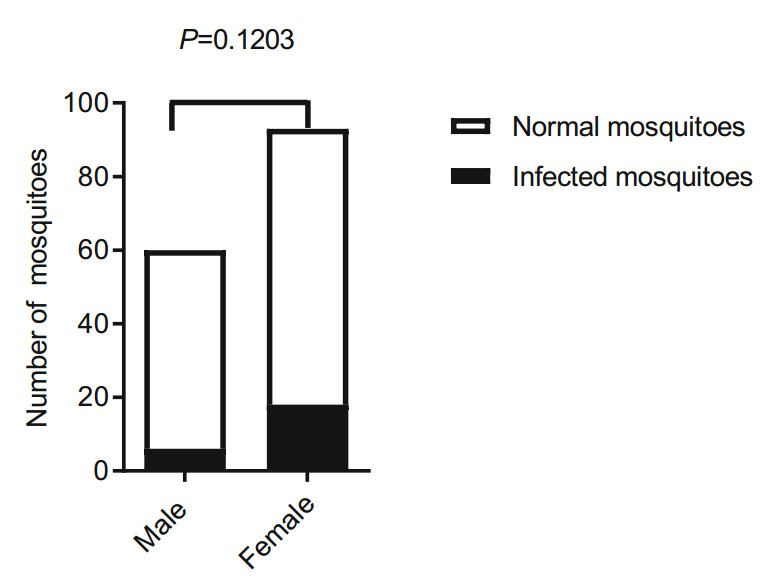
To characterize the viral kinetics of DENV, approximately 20 wintering mosquitoes were randomly collected every month from October 16, 2016, to June 15, 2017, and DENV-1 RNA was analyzed by qRT-PCR. The infection was detectable in the mosquitoes which sampled at different time points. The results indicated that the Ct value of most mosquito samples ranged from 28 to 40 and several were as high as 21. The proportion of DENV-1 positive mosquitoes ranged from 9% to 80% and decreased with prolonged sample collection time, but the positive rate remained unchanged over three months after the experiment was started (P < 0.05) (Fig. 2). DENV-1 RNA was detected in both male and female mosquitoes. The cumulative rates of DENV-1 positive mosquitoes in males and females were 10% and 19.3%, respectively, but there was no difference in positive rate between males and females (P = 0.1203) (Fig. 3).
-
The female mosquitoes fed on a litter of normal Kunming suckling mice (n = 9–13 per litter) for 2 h per 10 days for the duration of the study period and the ambient temperature of was recorded (Fig. 1A). Those females were categorized based on abdominal distension after a blood meal and were counted as engorged/non-engorged, and the percentage of engorged females was subsequently counted. The proportion of engorged female mosquitoes ranged from 5.2% to 91.3% when the temperature was between 15 ℃ and 30 ℃. No engorged females were observed when the temperature was below 15 ℃. Significant coherence was observed between the percentage of engorged females and the ambient temperature (R2 = 0.9351). For temperatures ranging from 15 ℃ to 30 ℃, a linear relation was observed between the percentage of engorged females and the ambient temperature (Fig. 4).
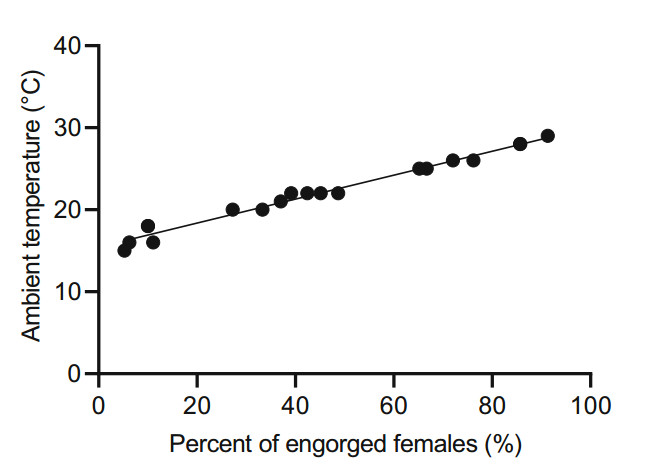
Figure 4. Percentage of engorged females on different blood meal dates. The positive correlation between the percentage of engorged females and the ambient temperature ranging from 15 to 30 ℃ with a coefficient of determination (R2) = 0.9351.
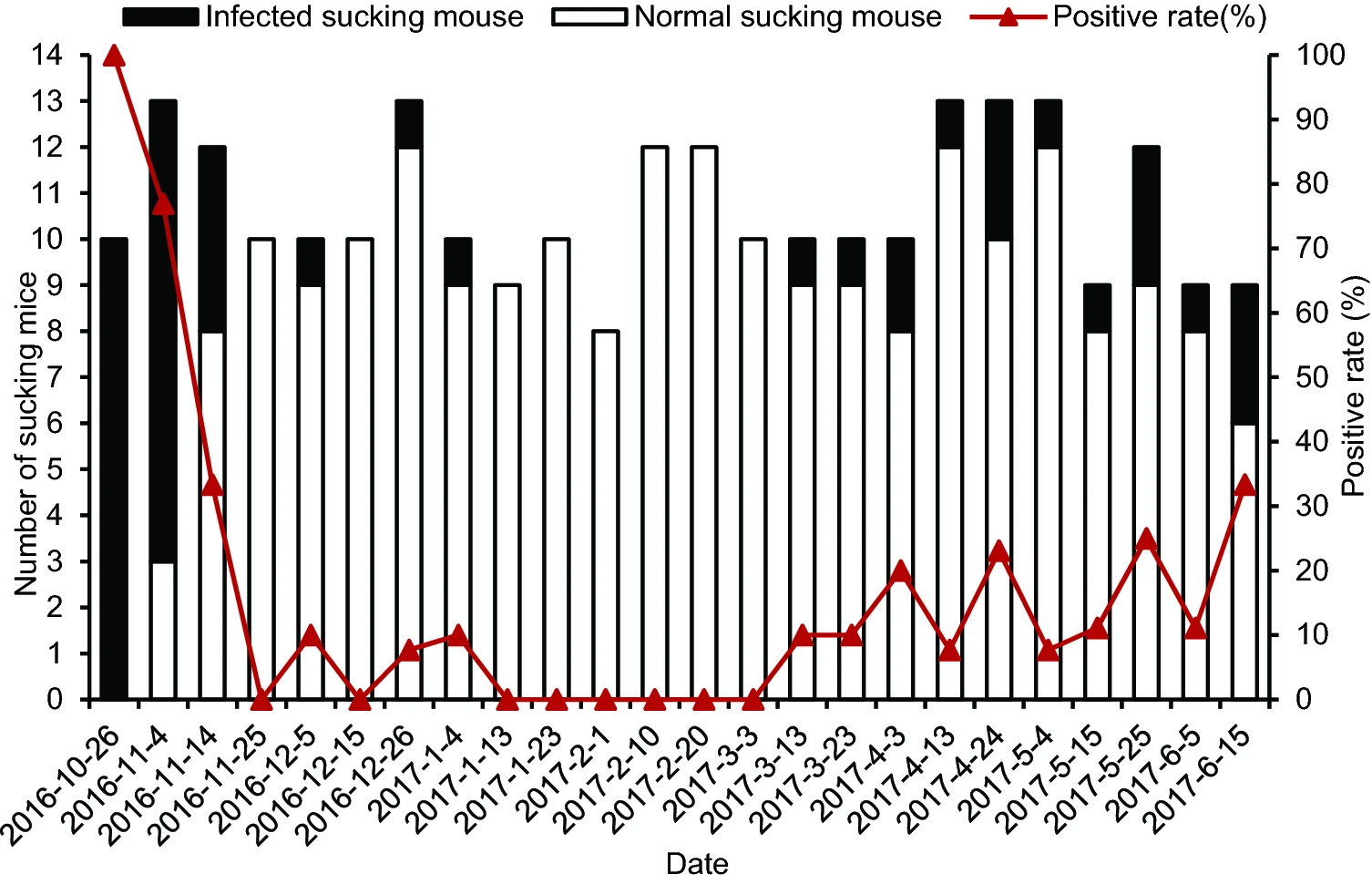
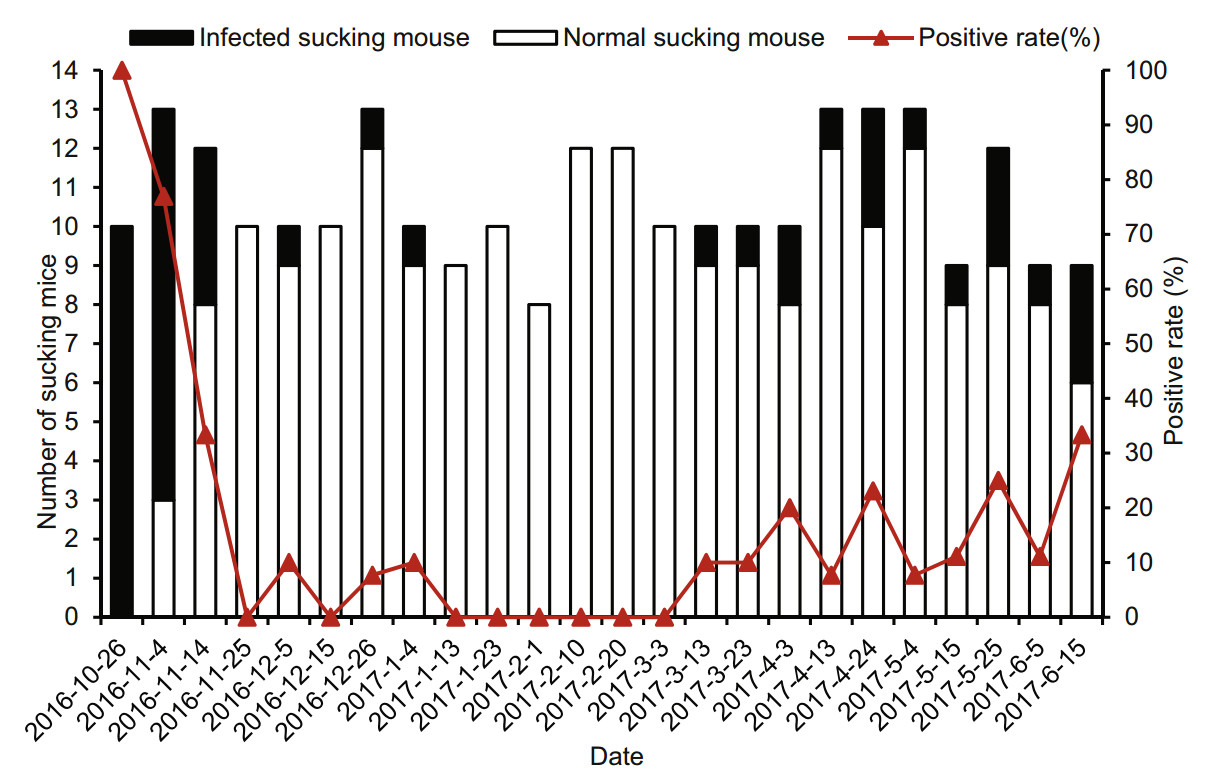
DENV was detected at a total positive rate of 18.8% in suckling mice bitten by mosquitoes on different days (Fig. 5). DENV-positive suckling mice bitten by mosquitoes were observed in 16 of the 24 groups. The DENV-1 positive rate of bitten suckling mice in those 16 groups ranged from 7.69% to 100%. Viraemia was detectable in all suckling mice bitten by mosquitoes on October 26, 2016, and in most (10/13) suckling mice bitten by mosquitoes on November 4, 2016, as well as in 33% (4/12) suckling mice bitten by mosquitoes on November 14, 2016. The DENV-1-positive rate of bitten suckling mice gradually decreased over time and with decreasing environment temperature from October 26 to November 14, 2016, and subsequently began to increase again. There was no difference in the DENV-1 positive rate of suckling mice bitten by mosquitoes from March 3 to June 16, 2017.
Variations in Temperature, Humidity and the Survival Rate of Mosquitoes
Viral Kinetics of DENV-1 Infection In Wintering Mosquitoes
Wintering Mosquitoes to Mouse Transmission
-
In recent decades, southern China, particularly the Pearl River Delta region, has become a dengue endemic region with the co-circulation of the four dengue virus serotypes and diseases caused by dengue virus (DENV) placing a serious burden on public health (Luo et al. 2017). Among the epidemiological characteristics of dengue outbreaks and the stably sustained serotypes and genotypes of DENV isolated in South China, whether DENV has been localized in South China has not been determined. A number of studies indicated that: dengue was still an imported disease from Southeast Asia and other regions (Sang et al. 2015; Sun et al. 2017); however, autochthonous human cases unrelated to imported cases have been reported (Luo et al. 2017; Yang et al. 2015). In this study, the dynamics of overwintering Ae. albopictus mosquitoes were observed, and the persistent vertical transmission characteristics of dengue virus in overwintering Ae. albopictus and the dynamic profile of DENV transmission by overwintering Ae. albopictus mosquitoes were comprehensively analyzed under a mock urban balcony ecosystem.
In the present study, Ae. albopictus eggs hatched, larvae developed and emerged, adults survived, fed on blood and produced eggs in winter in a semi-natural setting, and the number of adult mosquitoes was strongly affected by the environment, which implied that Ae. albopictus can live in winter in Guangzhou. Yang et al. (2020) also observed the egg hatching rate, larval development time, adult emergence rate and activity of Ae. albopictus adults under diurnal temperature conditions from August to next year July. Wu et al. (2019) monitored the density of Ae. albopictus in urban villages of Guangzhou from May to October 2017. Li et al. (2020) monitored Ae. albopictus distribution and population dynamics on Hainan Island, China. The findings described in these reports were consistent with our results and implied Ae. albopictus can also live in winter in subtropicaltropical areas of China (Zheng et al. 2019).
In this report, for the first time, we describe semi-natural vertical transmission of DENV in Ae. albopictus in Guangzhou. DENV was detected in overwintering Ae. albopictus collected at different time points during the 8-month experimental period. DENV-1 kinetics in the main vector of Guangzhou, Ae. albopictus, have been characterized. There were different proportions of DENV-1 positive mosquitoes ranging from 9 to 80% in the overwintering Ae. albopictus and DENV-1 RNA was also detected in male mosquitoes. Vertical transmission of DENV has been demonstrated in three consecutive generations of Ae. albopictus mosquitoes under laboratory conditions (Shroyer 1990) and was first observed in Aedes aegypti mosquitoes under natural conditions in Trinidad in 1984 (Hull et al. 1984). In subsequent decades, other published studies have reported the presence of natural vertical transmission of DENV in both males and females of Ae. albopictus in tropical areas (Ayllón et al. 2018; Ferreira-de-Lima et al. 2020; Ferreira-de-Lima and LimaCamara, 2018; Teixeira Mda et al. 2002; Tewari et al. 2004). Considering both these data and our result, it should be concluded that the natural vertical transmission of DENV may occur in overwintering mosquitoes in Guangzhou. Further studies are needed to measure a silent dengue transmission in overwintering mosquitoes under natural conditions. The genetic variants and genetic relationships of these strains in overwintering mosquitoes should be analyzed, and a prospective field survey should be conducted in endemic areas in Guangzhou.
In addition, the dynamic profile of the vector competence of DENV-1-positive overwintering Ae. albopictus was also analyzed by studying bitten suckling mice. A positive relationship between the DENV-1 positive rate of bitten suckling mice and the percentage of engorged females was reported in a previous study (Chen et al. 2019). Previously published studies have also shown that the biting behavior of female mosquitoes is among the crucially important factors affecting the vector competence of Ae. albopictus and are correlated with temperature, with no biting activity occurring below 11 ℃ or above 36 ℃ under laboratory conditions (Duan et al. 2017). In this study, no biting activity of Ae. albopictus was observed below 15 ℃ and the biting behavior of female mosquitoes were significantly correlated with temperature (primarily occurring between 15 ℃ and 30 ℃) under semi-natural conditions, which provides a helpful guideline for implementing practical strategies to achieve vector control.
This study elucidated the dynamics of the overwintering Ae. albopictus mosquitoes, the dynamic profile of dengue virus in overwintering Ae. albopictus and the vector competence dynamics of overwintering Ae. albopictus in a mock urban balcony ecosystem in Guangzhou, China. Our findings are important for the assessment of dengue transmission risks in winter and may facilitate the planning of mosquito control activities at the local and regional levels in subtropical areas.
-
This project was supported by the Provincial Science and Technology Projects of Guangdong Province, China (Grant numbers: 2014A020219007, 2015A020218003, 2016A020219006 and 2017A020215101) and the Medical Scientific Research Foundation of Guangdong Province, China (Grant numbers: A2018167). The funders had no role in the study design, data collection and analysis, decision to publish, and preparation of the manuscript.
-
YC and RR designed the study. YC, RC, JG, CL, JZ and JL collected samples and analyzed the data. YC, RC and RR wrote the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
All procedures were conducted in compliance with a protocol approved by the Animal Ethics Committee of the Center for Disease Control and Prevention of Guangzhou Military Command. All experiments were performed in accordance with relevant guidelines and regulations.















 DownLoad:
DownLoad: