-
Dear Editor,
Dogs were long considered refractory to influenza viruses until the equine-origin H3N8 canine influenza virus (CIV) was first isolated in the United States of America (U.S.A) in 2004 (Crawford et al. 2005). Since then, H3N8 CIV of equine-origin strains has been circulating in dogs in the U.S.A. In 2006, another CIV of avian origin H3N2 subtype was isolated from dogs in China (Li et al. 2010). Recently, H3N2 CIV strains have become endemic in dog populations in China, South Korea, and North America (Borland et al. 2020).
Subtype H3N8 equine influenza virus (EIV) was first identified in Florida in 1963 (Waddell et al. 1963). The reassortment has played a major role in the evolution of EIV (Murcia et al. 2011). Since approximately 1990, H 3N8 EIV has diverged into American and Eurasian lineages (Daly et al. 1996), and subsequently, the American lineage further diverged into Florida Clade 1 (FC1) and Florida Clade 2 (FC2) (Lai et al. 2004). FC1 viruses predominate in America herds, and FC2 is the predominant strain with an autochthonous monophyletic group among horses in China (Yang et al. 2018; Mino et al. 2019). Although no H3N8 EIV infection in dogs has ever been reported in China, the risk of FC2 H3N8 EIV moving from horses to dogs cannot be ignored. Hence, in this study, dogs were experimentally inoculated with FC2 H3N8 EIV for evaluation of the threat of this virus to dogs in China.
Twelve 9- to 11-week-old influenza antibody-negative beagles were used in this study. All animals were anesthetized with an intramuscular injection of ketamine (20 mg/kg) and xylazine (2 mg/kg) mixture before inoculation. As a negative control, four beagles (n = 4) were inoculated with pathogen-free phosphate-buffered saline (PBS). Two groups of four beagles (n = 4) were intranasally inoculated with 106 EID50 in 1.0 mL PBS of H3N2- CIV-02 (A/canine/Guangdong/02/2011, the enzootic subtype virus among dogs in China; as a positive control) or H3N8-EIV-Z (A/equine/Heilongjiang/Z/2010, the predominant strain among horses in China).
Fever, anorexia, conjunctivitis, diarrhea, labored breathing, cough, decreased activity were recorded daily at intervals of 24 h for 3 days before inoculation and 14 days post-inoculation (dpi). In the positive control group, the beagles showed clinical symptoms, including rhinorrhea and fever. Their body temperatures increased during the period from 1 to 4 dpi (Fig. 1A). In H3N8-EIV-Z group, all animals were healthy.
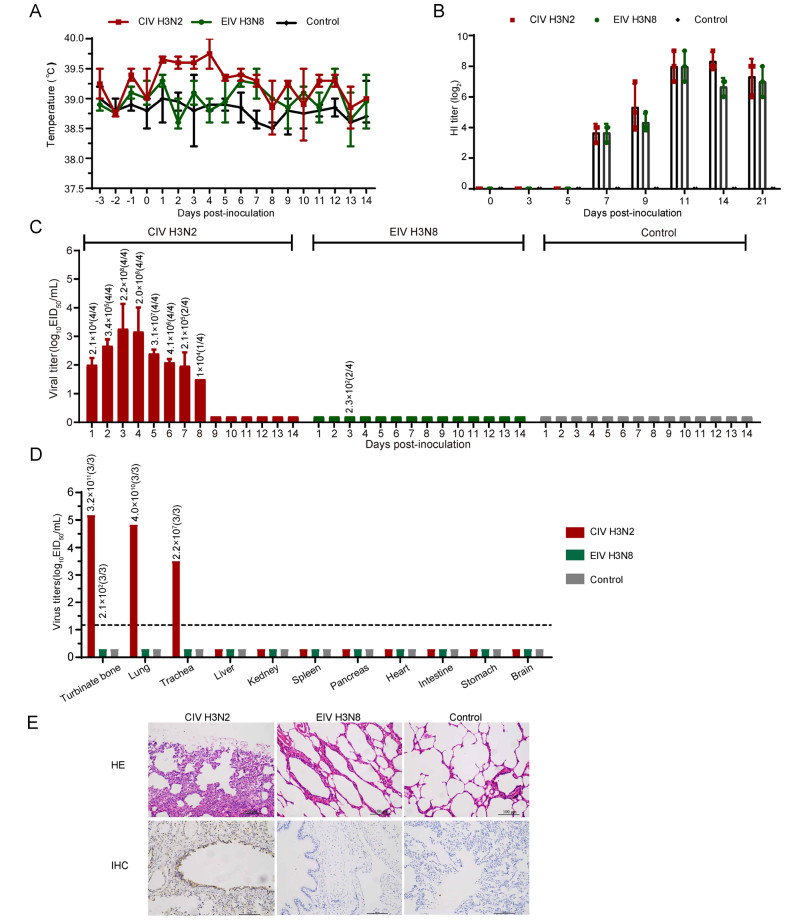
Figure 1. Experimental inoculation of Florida clade 2 H3N8 equine influenza virus and H3N2 canine influenza virus in Beagles. Dogs were inoculated with 106 EID50 of virus by intranasal inoculation. Rectal body temperature (A), and the viral titers (C) of nasal swabs were monitored for 14 days, the antibody (B) were detected on 0, 3, 5, 7, 9, 11, 14 and 21 dpi. Viral replication (D) and pathological changes (E) in the lungs were detected on 4dpi. Lung tissues were stained with HE stain and the antigen were detected by a mouse anti-influenza A nucleoprotein (NP) monoclonal antibody and HRP-labeled goat antimouse secondary antibody. Different colors represent different individuals: red is CIV H3N2 group, green is EIV H3N8, and grey is negative group; IHC: Immunohistochemical; HE: Hematoxylin–eosin. Magnification of the IHC and HE photos were ×200. Brownish staining indicates detection of viral antigen expression in IHC. The numbers above the columns in C and D represent the copies detected by qPCR from nasal swabs and tissues, and the numbers in brackets represent positive/total numbers of dogs.
Sera of all animals were collected on 0, 3, 5, 7, 9, 11, 14 and 21 dpi. Before the assays, all serum samples were treated with receptor destroying enzyme (RDE, prepared by the China National Influenza Center (CNIC)) and absorbed with erythrocytes to remove nonspecific inhibitors. We employed the chicken-RBCs HI assay to evaluate antibodies against avian influenza viruses. Antibodies were detected at 7, 9, 14, and 21 dpi in animals that were inoculated with H3N2-CIV-02 or H3N8-EIV-Z (Fig. 1B). No antibodies were detected in dogs of the negative control group (Fig. 1B).
The nasal and rectal swabs of all animals were collected daily from 1 to 14 dpi and placed into 1.0 mL of cold PBS with antibiotics and titrated by the EID50 assay in SPF chicken eggs and detected by absolute quantitative PCR (qPCR). On 4 dpi, one animal in each inoculation group was euthanized by intracardiac injection of pentobarbital sodium (150–200 mg/kg body weight). The turbinate bone, lung, trachea, liver, kidney, spleen, pancreas, heart, intestine, stomach and brain were collected from the euthanized animals for assessment of viral replication. For animal welfare, only one dog in each group was euthanized in this study, and three pieces of each tissue were collected. The tissues were weighed and homogenized in 1 mL/g of cold PBS, and the clarified supernatant was obtained by centrifuging, then it was titrated by an EID50 assay and was detected by qPCR. In H3N2-CIV-02 infection group, virus was detected in the nasal swabs of the beagles, and continued shedding from 1 to 8 dpi (Fig. 1C). Viral titers were detected in the lung, turbinate, and trachea of the euthanized beagles at 4 dpi but were not detected in other organs (Fig. 1D). In H3N8-EIV-Z group, low copies viruses were detected from nasal swabs on 3 dpi (Fig. 1C), and limited virus replication was detected in turbinate bone (Fig. 1D).
For histopathological examination, lungs were fixed in 10% neutralized phosphate-buffered formalin and subjected to hematoxylin and eosin (H & E) staining as well as immunohistochemistry (IHC). Briefly, for H & E staining: fixed tissues were dehydrated, embedded in paraffin, cut into 5-lm-thick sections, and stained with standard hematoxylin and eosin. For IHC: tissue sections were incubated with a mouse monoclonal antibody raised against the influenza A virus nucleoprotein (Sigma-Aldrich company), and then they were incubated with horseradish peroxidase (HRP) goat anti-mouse IgG(H? L) antibody (Abbkine company), and then stained with diaminobenzidine (DAB). Histologically, the lungs of the beagles appeared consistent with bronchial interstitial pneumonia, and there was massive recruitment of lymphocytes (HE) and antigen signs (IHC) (Fig. 1E). In H3N8-EIV-Z group, all animals were healthy. There was no pneumonia and no antigen signs in the lungs (Fig. 1E).
Dogs were long regarded as refractory to influenza viruses. However, the equine-origin H3N8 virus and the avian-origin H3N2 virus have been established among dogs (Xie et al. 2016). H3N8 CIV mainly circulates in North America, and H3N2 CIV mainly circulates in Asia. Additionally, H1N1, reassortment H3N1 and H3N2, and H5N1 were isolated from dogs and were pathogenic to dogs. Hence, dogs have come to be considered as a potential "mixing vessel" of influenza A viruses (Gonzalez et al. 2014; Zhu et al. 2015; Xie et al. 2016). The circulating H3N8 CIV is due to the interspecies transmission of H3N8 EIV by a reassortant virus from the circulating FC1 H3N8 EIV (He et al. 2019). Previous studies reported that H3N8 EIV infected pigs and donkeys in China (Tu et al. 2009; Qi et al. 2010). Besides, our previous study found seroepidemiological evidence of subtype H3N8 influenza virus infection among pet dogs in China (Zhou et al. 2016). The FC2 H3N8 EIV is the predominant strain among horses in China (Yang et al. 2018). Therefore, dogs are at potential risk from subtype H3N8 influenza virus in China, especially the FC2 H3N8 EIV. However, the beagles showed asymptomatic infection to FC2 H3N8 EIV in this experimental inoculation. Therefore, the potential for transmission has not been firmly established, but further surveillance and mechanism studies must be performed.
HTML
-
This project was supported in part by the National Natural Science Foundation of China (31802204, 31872454) and the Natural Science Foundation of Guangdong Province, China (2018B030311037, 2018A030313633).
-
The authors declare that they have no conflict of interest.
-
All institutional and national guidelines for the care and use of laboratory animals were followed. All procedures in the animal experiments met the requirements and were approved by the Experimental Animal Welfare Ethics Committee of the South China Agricultural University. Euthanasia was performed using an intravenous injection of pentobarbital. All experimental animals were monitored by university-licensed veterinarians. All animal experiments were performed in a level 2 animal biosafety laboratory (A-BSL level 2).
















 DownLoad:
DownLoad: