-
Dear Editor,
In the absence of vaccine, the mounting second wave of COVID-19 infections worldwide calls for the rapid development of human neutralizing antibodies to prevent and treat COVID-19 (Lee et al. 2020). Considering the high cost and long development cycle of human neutralizing antibodies, we turned to anti-SARS-CoV-2 IgY for cheap and fast scale up.
In particular, egg yolk antibody IgY, the evolutionary precursor of IgG, is widely present in birds, reptiles, and amphibians, and it can be easily obtained on a large scale from egg yolk (Pereira et al. 2019). As a component of chicken eggs consumed by humans, IgY is expected to be safe and has been widely applied as prophylactics against respiratory infections because of its high binding affinity to antigens, thermostability, high resistance to pH variation, and cost-effectiveness (Abbas et al. 2019). Because of the advantages of IgY, we aimed to isolate anti-SARS-CoV-2 IgY from the yolks of eggs delivered by hens immunized with inactivated SARS-CoV-2 and evaluate its inhibitory activity against SARS-CoV-2 infection in vitro, with a hope to develop IgY-based immunoprophylactic or therapeutic for prevention or treatment of COVID-19.
Specifically, SARS-CoV-2 (20SF014-SARS-CoV-2) was expanded in Vero-E6 cells, collected, and stored at -80 ℃ until use. Lohmann pink-shell laying hens were subcutaneously immunized with formaldehyde-inactivated SARS-CoV-2 and Freund∩s complete adjuvant, boosted twice at 2-3 week interval with the mixture of the inactivated virus and Freund∩s incomplete adjuvant on both wings (0.5 mL/hen). One week after the final immunization, the eggs were collected, and crude IgY antibodies were extracted from the egg yolks using the water-soluble fraction method (Supplementary Materials). Purified IgY antibodies were confirmed by Western blotting (Fig. 1A). We then tested the neutralizing activity of these IgY antibodies against pseudotyped and live SARS-CoV-2 infection in vitro as previously described (Xia et al. 2020). As shown in Fig. 1B, 1C, the IgY antibodies were effective in neutralizing infection by both pseudotyped and live SARS-CoV-2 in a dose-dependent manner with half maximal inhibitory concentration (IC50) of 9.26 and 44.31 μg/mL, respectively, while the isotype control IgY antibodies had no inhibitory effect on pseudotyped and live SARS-CoV-2 infection at a concentration as high as 100 μg/mL.
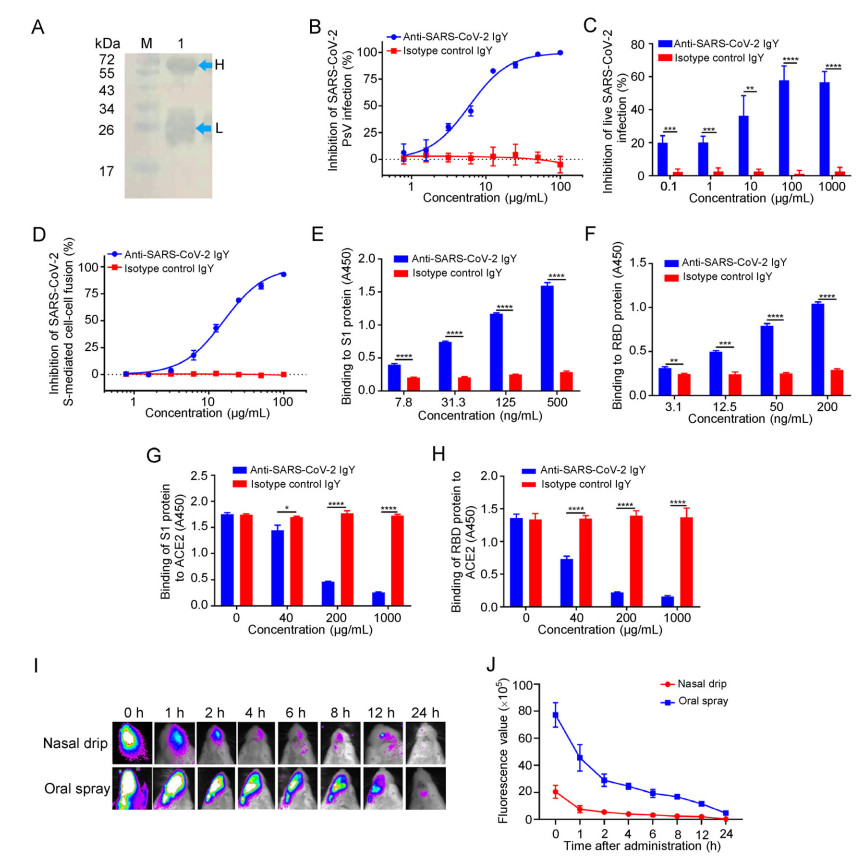
Figure 1. Inhibition of SARS-CoV-2 infection by IgY obtained from egg yolks of hens immunized with inactivated SARS-CoV-2. A Identification of anti-SARS-CoV-2 IgY antibodies purified from egg yolk by Western blot (Lane M: protein markers; Lane 1: IgY; H: heavy chain of IgY; L: light chain of IgY); B-D The inhibitory effect of anti-SARS-CoV-2 IgY antibodies on pseudotyped SARS-CoV-2 infection, as measured by luciferase assay (B), and live SARS-CoV-2 infection, as measured by cytopathic effect (C), as well as SARS-CoV-2 S-mediated cell-cell fusion, as measured by immunofluorescence assay (D). E, F The binding of anti-SARS-CoV-2 IgY antibodies to S1 protein (E) and RBD protein (F) was measured by ELISA. G, H Effect of anti-SARS-CoV-2 IgY antibodies on the binding of S1 protein (G) and RBD (H) to ACE2 was measured by ELISA. I, J Detection of anti-SARS-CoV-2 IgY in the nasal and oral cavities of mice was measured by in vivo imaging. Growth curves were obtained from serial measurements in three experiments (four or five animals per group). Experiments were repeated twice, and the data are presented as means ± SD. GraphPad Prism 5.0 was used to perform statistical analysis, and a P value of less than 0.05 was considered to indicate a significant difference, *P < 0.05; **P < 0.01; ***P < 0.001;****P < 0.0001.
Next, we assessed the inhibitory activity of purified IgY antibodies on SARS-CoV-2 spike (S) protein-mediated cell-cell fusion as previously described (Xia et al. 2020). As shown in Fig. 1D, these IgY antibodies were also effective in inhibiting SARS-CoV-2 S-mediated cell-cell fusion in a dose-dependent manner with IC50 of 15.83 μg/mL, while, again, no inhibitory activity was observed in the isotype control IgY group at a concentration as high as 100 μg/mL. These results suggest that IgY antibodies obtained from egg yolks of hens immunized with inactivated SARS-CoV-2 can effectively inhibit SARS-CoV-2 infection in vitro.
Jiang and colleagues have shown that the receptor-binding domain (RBD) in S1 subunit of SARS-CoV and SARS-CoV-2 S protein contains the major neutralizing epitopes and neutralizing antibodies from SARS-CoV- and SARS-CoV-2-infected patients, as well as hosts immunized with the inactivated vaccines that mainly target RBD (He et al. 2005, 2006; Du et al. 2009; Su et al. 2020). To illustrate the antiviral mechanism of anti-SARS-CoV-2 IgY antibodies, we performed an enzyme-linked immunosorbent assay (ELISA) to test whether these antibodies interact with S1 and RBD in S protein of SARS-CoV-2 and whether they can block the binding of S1 and RBD to human ACE2. As shown in Fig. 1E, 1F, anti-SARS-CoV-2 IgY antibodies bound to S1 protein and RBD in a dose-dependent manner, while isotype control IgY antibodies could not interact with either S1 or RBD. Notably, anti-SARS-CoV-2 IgY antibodies could significantly block the binding of S1 or RBD to ACE2 in a dose-dependent manner, while the isotype control IgY antibodies could not inhibit interaction between S1 or RBD and ACE2 (Fig. 1G, 1H). These results indicate that anti-SARS-CoV-2 IgY antibodies can bind to RBD in S1, thus blocking binding between RBD and ACE2 and inhibiting SARS-CoV-2 entry, fusion, and infection.
Ciliated nasal epithelial cells are particularly susceptible to SARS-CoV-2 infection owing to the high expression of ACE2, making the upper airways a key target for SARS-CoV-2 infection (Hou et al. 2020). Previous studies have shown that IgY passive immunization can be used to prevent and treat virus infection in the respiratory tract (Abbas et al. 2019). Therefore, to test whether anti-SARS-CoV-2 IgY antibodies could be used in nasal or oral spray formulation, we assessed the persistence time of anti-SARS-CoV-2 IgY in the nasal and oral cavity of mice by using an in vivo imaging system. To accomplish this, anti-SARS-CoV-2 IgY antibodies labeled with fluorescent molecules were administered to BABL/c mice via nasal drip or oral spray. At 0, 1, 2, 4, 6, 8, 12, and 24 h after administration, respectively, images were captured with the IVIS Imaging System. As shown in Fig. 1I, 1J, anti-SARS-CoV-2-IgY antibodies could persist at a detectable level in the nasal and oral cavities for 2-4 h and 12-24 h after administration, respectively. These results suggest that anti-SARS-CoV-2 IgY can stay in the upper airways for a matter of hours, depending on the administration method used.
In conclusion, the laboratory-cultured and formaldehyde-inactivated SARS-CoV-2 can be used to immunize hens for large-scale production of egg yolk IgY antibodies with potent inhibitory activity against live and pseudotyped SARS-CoV-2 infection in vitro, as well as SARS-CoV-2 S-mediated cell-cell fusion. In vivo imaging assay showed that anti-SARS-CoV-2 IgY antibodies administered via oral spray or nasal drip could stay in the upper airways for hours. Considering that IgY antibodies are safe for human use and can be manufactured on a large scale with low production cost, these anti-SARS-CoV-2 IgY antibodies have good potential to be further developed as an immunoprophylactic via nasal or oral spray administration to prevent COVID-19. This approach can also be adapted for the development of immunoprophylactics against influenza virus H7N9 using the IgY antibodies from egg yolks of chickens immunized with anti-H7N9 vaccine (Zeng et al. 2018), achieving double effects, i.e., prevention of H7N9 infection in chickens and humans.
HTML
-
This work was supported by grants from the Key Research and Development Project of Guangdong Province (202020012624900001), the National Natural Science Foundation of China (31830097, 31802174 and 82041025), the Key Research and Development Project of Guangdong Province (2020B1111320001), and the Special fund for the Scientific innovation strategy-construction of high level Academy of Agriculture Science-Prominent Talents (R2020PY-JC001).
-
The authors declare that they have no conflict of interest.
-
The mouse experiments were carried out in strict accordance with institutional regulations (approved by the Ethics Committees of the Institute of Process Engineering, Chinese Academy of Sciences, No. 20200610-1).

















 DownLoad:
DownLoad: