HTML
-
The mosquito-borne virus Japanese encephalitis virus (JEV) belongs to the Flaviviridae family (Aguirre et al. 2012; Barrows et al. 2018). In Asia, JEV causes ~68, 000 cases of viral encephalitis each year in humans, with mortality rate ranging from 25% to 40%. More than 50% of the survivors will suffer from severe neurological sequelae (Campbell et al. 2011; Solomon 2006). Unfortunately, there are still no specific and effective antiviral drugs to treat JEV infection. Like other flaviviruses, JEV is an enveloped virus, which contains a single-stranded positivesense RNA genome of roughly 11 kb. The viral polyprotein is cleaved into three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) by virus or hostencoded proteases (Neufeldt et al. 2018; Slon Campos et al. 2018). In addition, flavivirus NS2B-3 is a protein complex composed of NS2B and NS3. The NS3 protein is an important enzyme with the serine protease activity of N-terminus and the helicase activity of C-terminus (Lei et al. 2016; Li et al. 2019). The NS2B protein is a cofactor of NS3, which is essential for the localization and activation of NS3 (Xing et al. 2020). NS2B-3 plays important roles in flaviviruses replication cycle, including cleavage of viral precursor polyproteins and inhibition of host immunity (Chen et al. 2017; Ding et al. 2018). Some studies have reported that NS2B-3 can degrade host proteins through the ubiquitination pathway to facilitate persistent and productive viral replication (Li et al. 2019; Liu et al. 2017).
AXL, one member of the TAM family, plays pivotal roles in diverse biological processes with other two members Tyro3 and Mertk. TAM and their cognate ligands Gas6 and protein S are crucial for macrophage and dendritic cells to phagocytize apoptotic cells (APs) through binding to phosphatidylserine (PS), which is displayed on the surface of APs (Lemke 2019; Rothlin et al. 2015). They also function as pleiotropic inhibitors of inflammatory responses to various pathogens in the immune system (Lemke 2013; Rothlin et al. 2007). In addition, AXL was identified as an entry receptor for several flaviviruses. Dengue virus (DENV) and multiple pseudotyped enveloped viruses expose the PS on their membranes, through which they bind Gas6 and activate the AXL receptor (Bhattacharyya et al. 2013; Meertens et al. 2012). Studies on glial cell (Retallack et al. 2016), placental cells (Tabata et al. 2016), and sertoli cells (Kumar et al. 2018; Strange et al. 2019) have shown that AXL is a potential entry receptor for Zika virus (ZIKV). However, knocking out AXL does not prevent ZIKV entry and ZIKV-induced death in human neural progenitor cells (Hastings et al. 2017; Wang et al. 2017). Genetic ablation of AXL does not protect mice from ZIKV infection (Hastings et al. 2019; Wells et al. 2016). AXL inhibits the neuroinvasion of JEV by reducing IL-1α production from pyroptotic macrophage (Wang et al. 2020). These results illustrate the complex functions of AXL in the flavivirus replication process.
Here, we found JEV NS2B-3 could substantially downregulate the expression of AXL in JEV infected cells through a proteasome-dependent pathway. The reduction of AXL protein led to cell apoptosis by blocking the phosphatidylinositol 3-kinase (PI3K)/Akt cascade reaction and promoted the release of more virus particles.
-
Human embryonic kidney 293T cell line HEK-293T, baby hamster kidney cell line BHK-21, and human brain microvascular endothelial cell line hBMEC were grown in Dulbecco's modified Eagle's medium (DMEM, 11965092, GIBCO, Carlsbad, CA, USA). Adenocarcinomic human alveolar basal epithelial cell line A549 was grown in RPMI 1640 (31870082, GIBCO), containing 10% fetal bovine serum (FBS, 10100, GIBCO) and 1% penicillin– streptomycin. 293T, A549, and BHK-21 cells were preserved in our laboratory. hBMEC cells were kind courtesy of Dr. Zhe Ma (Nanjing Agricultural University, Nanjing, China). All cells were cultured at 37 ℃ with 5% CO2.
-
JEV NJ2008 (GenBank: GQ918133.2), SA14-14-2 (GenBank: MK585066.1) and HN07 (GenBank: FJ495189.1) strains were proliferated in C6/36 (Aedes albopictus cell line) cells or BHK-21 cells. The parent strain of JEV HTB is JEV HN07 strain. The 24th nucleotide of NS2A gene of JEV HTB was mutant artificially from T to A, and it did not express NS1' protein. Compared with JEV SA14-14-2 strain, JEV STB had a G to A mutation in the 66th nucleotide of NS2A gene, which expressed NS1' protein. Duck Tembusu virus (DTMUV) XZ2012 (GenBank: KM188953.1) strain was proliferated in BHK-21 cells. The titers of the virus were titrated on BHK-21 cells by plaque assays.
-
The gene encoding open reading frame of AXL was cloned into pcDNA3.1 (-). Gene of JEV NJ2008 strain capsid (1–127), premembrane (128–294), envelope (295–794), NS1 (795–1146), NS2B (1374–1504), NS3 (1505–2123), NS5 (2528–3433), prM-E (128–794), NS2B-3 (1374–2123) were inserted into p3 × FLAG-CMV-7.1. NS2B-3 deletion mutants were also inserted into p3 × FLAG-CMV-7.1. Note that p was used to represent plasmid in the following text.
Primary antibodies used in this study were listed as follow: JEV NS3 protein rabbit polyclonal antibody (GTX125868, GeneTex, CA, USA), human AXL protein rabbit monoclonal antibody (C89E7, Cell Signaling Technology, Boston, USA), human Akt protein rabbit polyclonal antibody (9272S, Cell Signaling Technology), and human p-Akt protein rabbit polyclonal antibody (9271S, Cell Signaling Technology), human AXL protein mouse monoclonal antibody (sc-166268, SantaCruz, Dallas, USA), PI3K protein rabbit monoclonal antibody (4257, Cell Signaling Technology), p-PI3K protein rabbit polyclonal antibody (PA5-38905, Invitrogen, Carlsbad, CA, USA), GAPDH protein mouse monoclonal antibody (AC033, ABclonal, Wuhan, China), mouse AXL protein goat polyclonal antibody (AF854-SP, R & D systems, Minnesota, USA), FLAG-tag mouse monoclonal antibody (66008-3-Ig, Proteintech, Wuhan, China), MYC-tag rabbit polyclonal antibody (16286-1-AP, Proteintech), HA-tag rabbit polyclonal antibody (51064-2-AP, Proteintech), and FLAG-tag goat polyclonal antibody (ab1257, abcam, Cambridge, UK). Secondary antibodies used in this study were listed as follow: donkey anti-goat IgG Alexa Fluor 594 (ab150132, abcam), donkey anti-mouse IgG Alexa Fluor 488 (ab150105, abcam), donkey anti-rabbit IgG Alexa Fluor 647 (ab150075, abcam), goat anti-rat IgG Alexa Fluor 555 (A-21434, Invitrogen), goat anti-rabbit IgG Alexa Fluor 488 (A-11008, Invitrogen), goat antirabbit IgG-HRP (31460, Invitrogen), goat anti-mouse IgGHRP (31430, Invitrogen), and rabbit anti-goat IgG-HRP (abs20005, absin, Shanghai, China).
Commercial reagents used in this study were listed as follow: recombinant human Gas6 peotein (DFGX05181102, R & D systems), GI254023X (abs814500, absin), TAPI0 (5523, TOCRIS, Bristol, UK), MG132 (S2619, Selleck, Houston, USA), control siRNA (sc-37007, SantaCruz), AXL siRNA (sc-29769, SantaCruz), FITC Annexin V apoptosis detection Kit (556547, BD, North Carolina, USA), and one-step TUNEL apoptosis assay kit (C1086, Beyotime, Shanghai, China).
-
A549 cells at approximately 80% confluence were transfected with AXL siRNA (siA). Similarly, AXL expression plasmids (pAXL, pAXL-D9) were transfected into 293T cells using Lipofectamine 3000 (L3000015, Invitrogen) for 24 h. For attachment assay, A549 or 293T cells were challenged with JEV at a multiplicity of infection (MOI) of 10 at 4 ℃. One hour later, the cells were washed three times with cold phosphate-buffered saline (PBS). For internalization assay, A549 or 293T cells were incubated with JEV at an MOI of 10 for 1 h at 4 ℃, and were washed for three times with cold PBS. Next, cells were moved to 37 ℃ for 30 min, and treated with proteinase K. For propagation assays, cells were inoculated with JEV at an MOI of 1 in FBS-free medium at 37 ℃ with or without Gas6. Two hours later, the medium was supplanted with new DMEM with supplemented 2% FBS. All samples were analyzed by Western blot or qRT-PCR.
-
RNA was extracted with Trizol and then transcribed into complementary DNA (cDNA). The cDNA templates were determined by real-time PCR using TB Green Premix Ex Taq (TliRNaseH Plus) (Takara, RR420Q, Dalian, China) and specific primers (see Supplementary Table 1).
-
Cells were lysed in RIPA buffer (89900, ThermoFisher, Waltham, USA) with protease and phosphatase inhibitors for 20 min at 4 ℃. Equal amounts of proteins were subjected to SDS-PAGE, and were transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were incubated with primary monoclonal antibodies overnight at 4 ℃ after being blocked with 5% nonfat milk for 3 h at room temperature (RT). Next, the membranes were incubation with horseradish peroxidase (HRP)- conjugated IgG secondary antibodies. Protein levels were visualized with BIO-RAD Clarity Western ECL Substrate. Quantification of the gray density of protein bands were quantified by ImageJ software. Please refer to the antibody catalogue for the primary and secondary antibodies used in WB experiments.
-
For coimmunoprecipitation assay, 293T cells were cotransfected with pAXL and pFLAG-NS2B-3, pFLAGNS2B, pFLAG-NS3 or pFLAG-NS2B-3 (H). After 24 h, MG132 (10 μmol/L) was added into cells for 18 h. Cells were lysed with NP40 buffer (P0013F, Beyotime) containing PMSF at 4 ℃ for 1 h. The whole lysate was incubated with protein A/G agarose beads (sc-2003, SantaCruz) for 1.5 h at 4 ℃, and after that was centrifuged at 1000 × g for 10 min at 4 ℃. The supernatants were incubated with anti-FLAG, anti-NS3, or anti-human AXL antibodies overnight. Antibody-added supernatants were treated with protein A/G agarose beads for 4 h at 4 ℃ and then collected by centrifugation (1000 × g for 5 min). Next, the immunocomplexes were washed for four times with NP40, and then suspended in lysis buffer for WB analysis. For ubiquitination assay, 293T cells were cotransfected with pAXL, pHA-ubiquitin and pFLAGNS2B-3/NS2B/NS3. After 24 h of transfection, MG132 (10 μmol/L) was added into cells for 12 h. The next operation steps were the same as above. Finally, the precipitated proteins were separated on SDS-PAGE and detected by WB.
-
A549 cells were infected with JEV at an MOI of 1, or cotransfected with pFLAG-NS2B-3, pFLAF-NS2B and pMYC-NS3 for indicated time points. Cells were fixed with 4% paraformaldehyde (20 min, at RT) and permeabilized with 0.1% Triton X-100 (10 min, at RT). For JEV infection, cells were incubated with anti-NS3, and antihuman AXL antibodies at 4 ℃ overnight, followed by incubation with goat anti-rat IgG Alexa Fluor 555, or goat anti-rabbit IgG Alexa Fluor 488 in the dark at 37 ℃ for 1 h. For plasmids transfection, cells were incubated with anti-human AXL, anti-FLAG, or anti-MYC antibodies at 4 ℃ overnight, followed by incubation with donkey antigoat IgG Alexa Fluor 594, donkey anti-mouse IgG Alexa Fluor 488, or donkey anti-rabbit IgG Alexa Fluor 647 in the dark at 37 ℃ for 1 h. Finally, the nucleus was stained with DAPI at RT for 5 min.
-
A549 cells were transfected with empty plasmid, pFLAGNS2B, pFLAG-NS3, and pFLAG-NS2B-3 or infected with JEV for 48 h in the absence or presence of Gas6. Then these cells were stained with the one-step TUNEL apoptosis assay kit according to the instructions.
-
A549 cells were transfected with indicated concentration of empty plasmid or pFLAG-NS2B-3 for 48 h in the absence or presence of Gas6, followed by staining with FITC Annexin V apoptosis detection Kit, and analyzed by flow cytometry.
-
BHK-21 cells on 6-well plates with 100% confluency were washed with fresh DMEM and incubated with 120 μL of tenfold serially diluted viruses at 37 ℃ for 2 h. Cells were then overlaid with 2 × DMEM (12100046, GIBCO) supplemented with 4% FBS and 1% low-melting-point agarose, and then cultured for 3–4 days. Next, cells were overlaid with 1% crystal violet in methanol for 6 h at RT.
-
The 4-week-old BALB/c mice were euthanized and disinfected with 75% ethanol, followed by intraperitoneal (i.p.) injection with 5 mL of DMEM. After 5 min massaged peritoneum and 10 min incubation, the DMEM in the peritoneum cavity was collected and then centrifuged at 400 × g for 10 min. The cell pellet was resuspended and cultured in DMEM with 10% FBS. For JEV infection, the peritoneal macrophages were infected with JEV HN07 strain at an MOI of 1.
-
All data were analyzed using an unpaired two-tailed t test and expressed as the mean ± standard error of mean (SEM). A P value ≤ 0.05 was considered statistically significant.
Cell
Virus
Plasmids, Antibodies and Reagents
Virus Attachment, Internalization, and Propagation Assays
Quantitative RT-PCR
Western Blotting (WB)
Coimmunoprecipitation and Ubiquitination Assays
Confocal Microscopy
TUNEL Staining
Flow Cytometry
Plaque Assays
Isolation of Primary Cells
Statistical Analysis
-
Several reports have identified AXL as a receptor and/or immune modulator for enveloped viruses in a cell typespecific manner, such as ZIKV (Strange et al. 2019). To investigate the biological significance of AXL during JEV infection, we tested the expression of AXL in four different cell lines. A549, HeLa, and hBMEC cells stably expressed AXL, while 293T cells did not (Fig. 1A). Therefore, A549 and HeLa cells were used as cell models for RNA interference experiments, and 293T cells were used for gene overexpression. When conducting JEV infection experiments, JEV NJ2008 strain produced in C6/36 cells was used firstly. Interestingly, endogenous AXL protein expression in A549 cells was significantly down-regulated with a time dependent manner after JEV infection (Fig. 1B top, Supplementary Fig. S1A). Previous studies have reported that flaviviruses produced in mammalian cells and insect cells are different in their ability to infect host cells by using AXL (Richard et al. 2017). To avoid this difference, we infected A549 cells with JEV NJ2008 strain which was produced in BHK-21 cells, and harvested cell lysates at different time points post-infection (Fig. 1B bottom, Supplementary Fig. S2B). Our data showed that endogenous AXL protein expression was also strongly down-regulated after JEV infection with a time-dependent manner (Fig. 1B, Supplementary Fig. S1A, S1B). The expression of AXL in A549 cells was down regulated more quickly in the group inoculated with JEV produced in C6/ 36 cells compared to that produced in BHK-21 cells. Conversely, AXL mRNA levels gradually increased in JEV infected A549 cells (Fig. 1C). Furthermore, we repeated the same experiments in HeLa cells and got the same results (Fig. 1D, 1E, Supplementary Fig. S1C), which suggested that the down-regulation of AXL mediated by JEV was not cell specific.
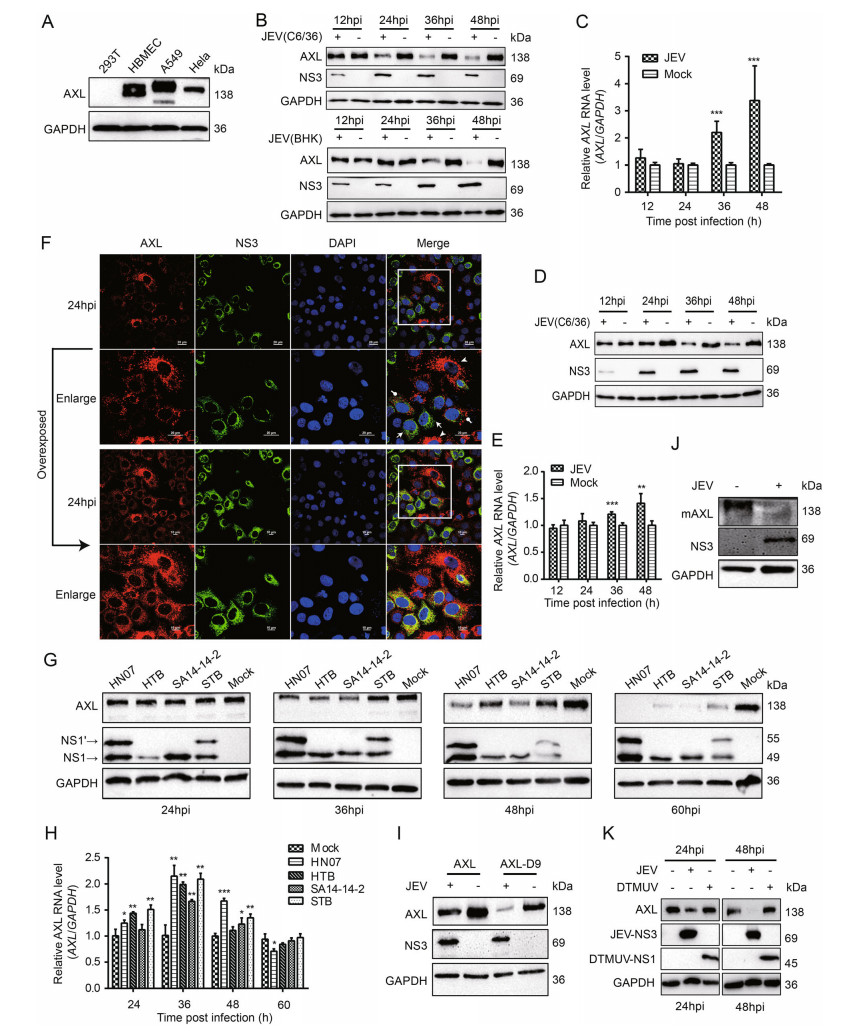
Figure 1. JEV degrades AXL at the protein level in vitro. A The ▶ expression of human AXL (hAXL) in different cells. B and D A549 (B) and HeLa (D) cells were inoculated with JEV (MOI = 1) at different time courses. The protein level of AXL was analyzed by WB. C and E A549 (C) and HeLa cells (E) were inoculated with JEV (NJ2008, MOI = 1), and the AXL RNA was detected by qRT-PCR. F A549 cells were infected with JEV (NJ2008, MOI = 1). After indicated hours post infection (hpi), cells were oberseved by confocal microscopy; scale bars, 10 lm; arrows and dots indicate JEV infected cells while arrowheads indicate JEV uninfected A549 cells. G and H A549 cells were inoculated with four different JEVs (HN07, HTB, SA14-14-2, and STB) at an MOI of 1 for different time courses. The level of AXL protein (G) was analyzed by WB. AXL RNA (H) was detected by qRT-PCR. I 293T cells were transfected with pAXL or pAXL-D9, and then infected with JEV (NJ2008, MOI = 1). The cell lysates were determined using WB. J Primary peritoneal macrophages were isolated from 4-week-old BALB/c mice, and they were challenged with JEV (HN07, MOI = 1) for 24 h. The AXL protein level was analyzed by WB using a murine-AXL antibody. K A549 cells were challenged with DTMUV and JEV (positive control) at an MOI of 1 for 24 or 48 h. The level of AXL protein was analyzed by WB. The results of three independent experiments are shown in the supplementary materials in the form of histograms with bar. All qPCR date were expressed as the mean ± SD of three independent experiments. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001. MOI, multiplicity of infection; WB, Western blotting; mAXL, murine AXL; JEV, Japanese encephalitis virus; DTMUV, duck Tembusu virus; SD, standard deviation.
To further explore the regulating role of JEV in AXL expression, we used fluorescence confocal microscopy to observe the protein level of AXL in A549 cells during JEV infection. We also found a potent decrease of AXL protein in JEV infected cells (Fig. 1F). In the Japanese encephalitis serological group viruses, there is a unique NS1 related protein NS1' that is produced due to the occurrence of programmed -1 ribosomal frameshift. The live attenuated JEV vaccine strain SA14-14-2 does not express NS1' due to the lack of GC-rich stable pseudoknot (Wang et al. 2015). Currently, there are five genotypes of JEV, which have different geographic distributions in Asia. Genotype I (GI) and genotype III (GIII) are the main strains circulating in China (Fan et al. 2019). In order to explore whether the down-regulation of AXL expression in JEV infected cells was virus strain specific, we detected the expression of AXL in cells infected with four different JEVs. JEV HN07 and HTB are belong to GI that differentially express NS1' protein, while JEV SA14-14-2 and rSA14-14-2 A66G are belong to GIII that differentially express NS1' protein. Cells infected with all these four JEVs led to a decreased protein level of AXL with a time-dependent manner (Fig. 1G, Supplementary Fig. S1D). Meanwhile, AXL mRNA levels gradually increased, which was similar to JEV NJ2008 strain group, and then became consistent with the mock group (Fig. 1H). These data indicated that different JEV strains and genotypes could decrease AXL protein expression in infected cells.
To further explore whether JEV could down-regulate exogenous AXL expression, we constructed two AXL eukaryotic expression vectors: pAXL (894 aa, GenBank: EAW57023.1) and pAXL-D9 (885 aa, GenBank: EAW57022.1). 293T cells were transfected with pAXL and pAXL-D9, and then infected with JEV. The results demonstrated a strong down-regulation of AXL protein expression by JEV infection (Fig. 1I, Supplementary Fig. S1E). Furthermore, primary peritoneal macrophages were isolated from wild-type mice and infected with JEV (HN07 strain) at an MOI of 1. We found the decrease of murine-AXL (mAXL) expression in JEV infected cells was also significant (Fig. 1J, Supplementary Fig. S1F). Furthermore, we used DTMUV, another flavivirus causing severe egg-drop and encephalitis in domestic waterfowl, to infect A549 cells and found that DTMUV infection did not down-regulate AXL protein expression (Fig. 1K, Supplementary Fig. S1G). In summary, these results indicated that AXL was a specific target inhibited by JEV.
-
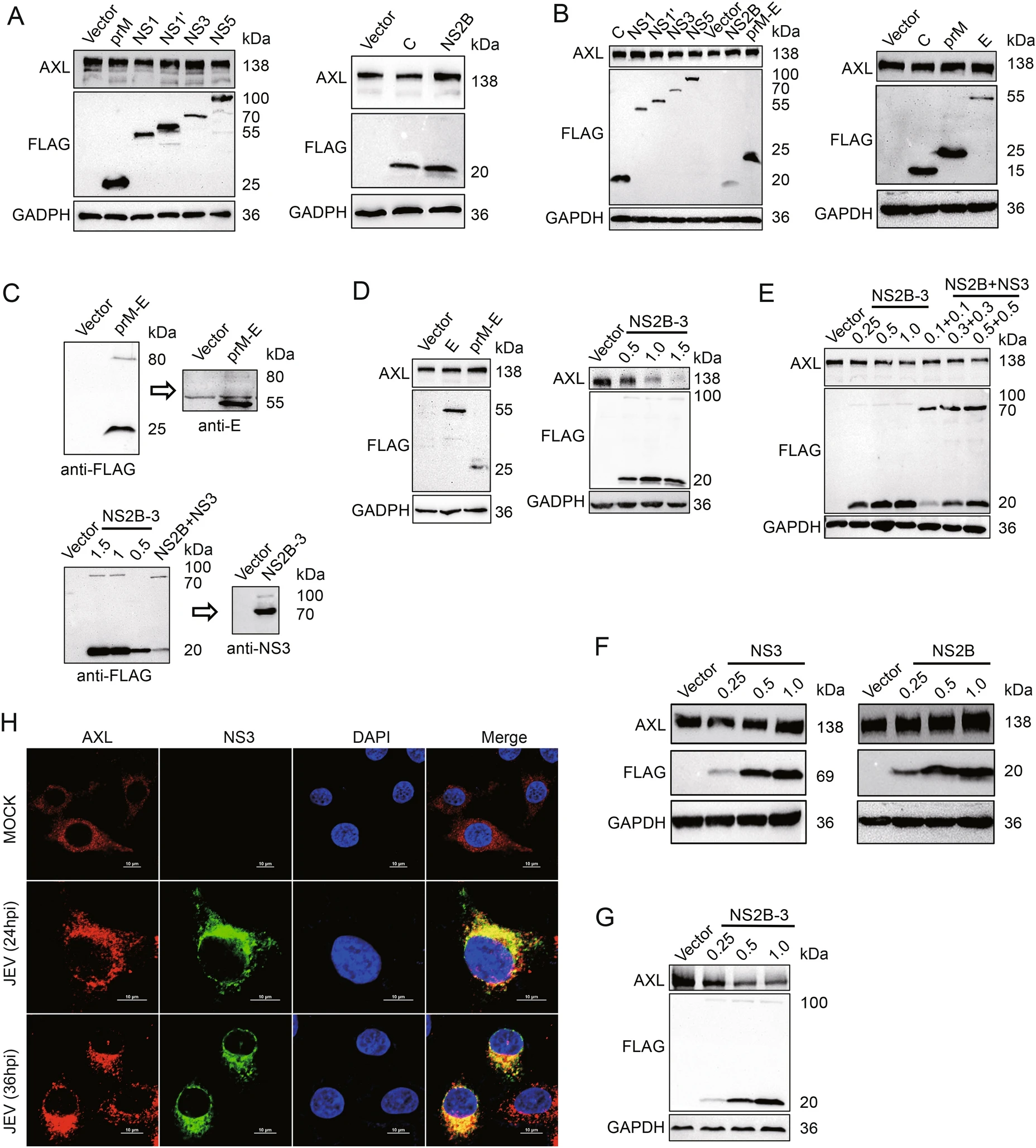
To explore the pathway of AXL protein expression regulated by JEV, JEV structural proteins C, prM, E and nonstructural proteins NS1, NS1', NS2B, NS3, NS5 were individually expressed in A549 and HeLa cells. None of these JEV-encoded proteins down-regulated AXL expression (Fig. 2A, 2B, 2D left panel lane 2, Supplementary Fig. S2A, S2B). During the viral replication cycle, prM and E form heterodimers to protect the fusion loop of E protein from premature fusion in the immature particles (Slon Campos et al. 2018). NS2B-3 protein complex, formed by NS3 and its cofactor NS2B, has been reported to be able to degrade a various of host proteins (Morrison et al. 2012). Therefore, we generated prM-E and NS2B-3 eukaryotic expression plasmids. The prM-E and NS2B-3 gene were cloned into p3 × FLAG-CMV-7.1 to obtain two constructs in which the three FLAG tags were in the N-terminal region. 293T cells were transfected with these two recombinant plasmids, then samples were checked by WB with FLAG, NS3 or E antibodies. Obviously, FLAG-prM-E and FLAG-NS2B-3 expressed in cells were mostly cleaved into FLAG-prM and E or FLAG-NS2B and NS3 (Fig. 2C). Then, we transfected A549 cells with pFLAG-prM-E or pFLAG-NS2B-3. JEV NS2B-3 protein complex, but not prM-E, could decrease AXL protein expression (Fig. 2D, Supplementary Fig. S2C). Additionally, NS2B-3 also downregulated endogenous AXL protein in HeLa cells with a dose-dependent manner (Fig. 2E lane 1 to 4, Supplementary Fig. S2D). The results of HeLa cells cotransfected with pNS2B and pNS3 were similar with that transfected with pNS2B-3 (Fig. 2E lane 1 and 5–7, Supplementary Fig. S2D). However, transfecting pFLAG-NS2B or pFALGNS3 individually into HeLa cells could not down-regulate the protein level of AXL (Fig. 2F, Supplementary Fig. S2E). Moreover, overexpressed JEV NS2B-3 decreased exogenous AXL protein in 293T cells with a dose-dependent manner (Fig. 2G, Supplementary Fig. S2F). These results indicated that AXL protein expression could be down-regulated only when both NS2B and NS3 were present, but not necessarily in the dimer form of NS2B-3.
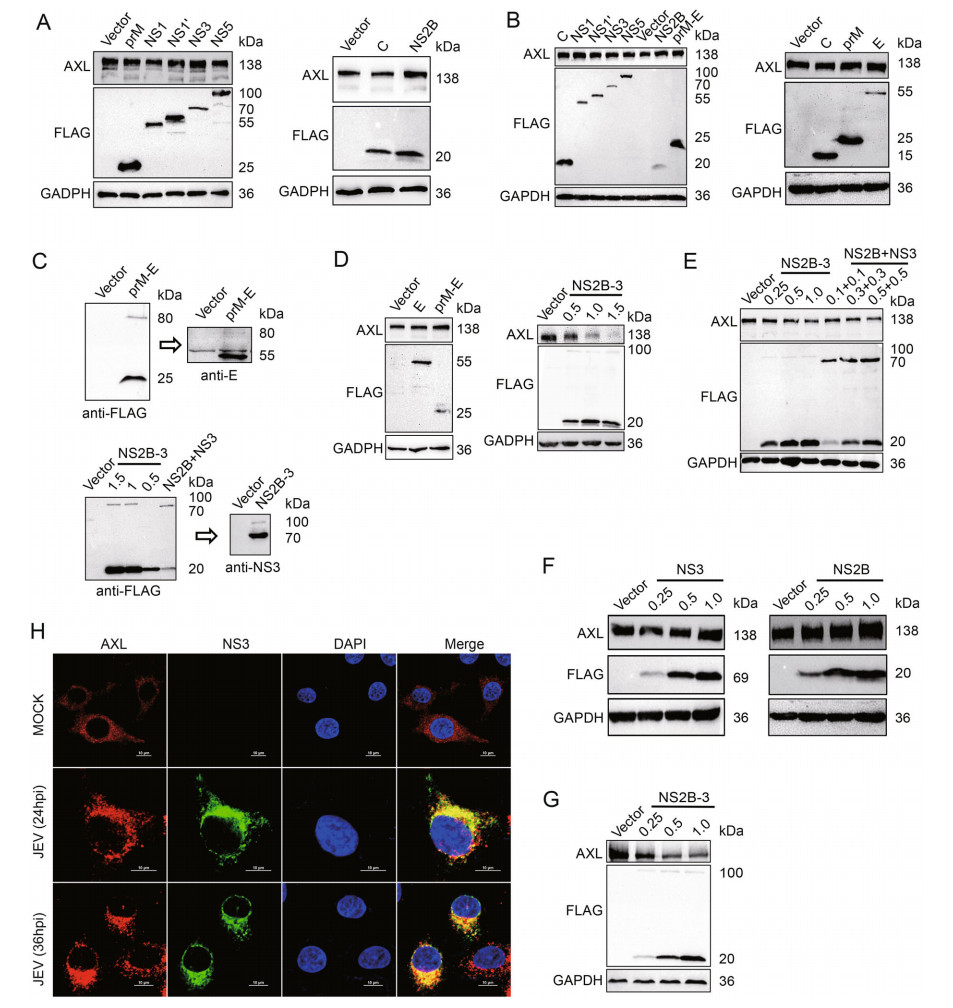
Figure 2. JEV NS2B-3 degrades AXL in the protein level. A and D (left panels) A549 cells were transfected with plasmids expressing JEV structural proteins (C, prM, and E) or nonstructural proteins (NS1, NS1', NS2B, NS3, and NS5). The AXL protein expressed at 48 h post transfection was analyzed by WB. B HeLa cells were transfected with plasmids expressing JEV structural proteins (C, prM, and E) or nonstructural proteins (NS1, NS1', NS2B, NS3, and NS5). After 48 h, the level of AXL was measured using WB. C p3 × FLAG-CMV-7.1- prM-E and p3 × FLAG-CMV-7.1-NS2B-3 (μg) were transfected into 293T cells. Anti-FLAG or anti-NS3 antibodies was used to test the related protein expression. D (right panels) A549 cells were transfected with different amounts of pFLAG-NS2B-3 (μg) for 48 h, and the AXL protein level was determined using WB. E HeLa cells were transfected with different amounts of pFLAG-NS2B-3 (lane 1 to 4) (μg) or cotransfected various amounts of pFLAG-NS2B and pFLAG-NS3 (lane 5 to 7) (μg). The expression of AXL in HeLa cells was analyzed by WB. F A549 cells were transfected with increasing amounts of pFLAG-NS2B (μg) or pFLAG-NS3 (μg). After 48 h, the cells were analyzed using WB. G 293T cells were transfected with pAXL (500 ng) along with increasing amounts of pFLAG-NS2B-3 (μg) for 48 h. Immunoblot analysis was performed with indicated antibodies. H A549 cells were challenged with JEV (NJ2008, MOI = 1) for indicated times, and the samples were analyzed with confocal microscopy. Red represents AXL, green represents JEV NS3, blue represents nucleus; scale bars, 10 μm. The results of three independent experiments are shown in the supplementary materials in the form of histograms with bar. WB, Western blotting; JEV, Japanese encephalitis virus; MOI, multiplicity of infection.
-
To explore the interaction between AXL and NS2B-3, confocal microscopy assays were performed to analyze the distribution of AXL and NS2B-3. We found that JEV NS3 protein extensively colocalized with AXL (Fig. 2H). Because NS2B-3 protein was mostly cleaved in host cells (Fig. 2C), we generated a pMYC-NS3 expression plasmid to distinguish from pFLAG-NS2B (Fig. 3A). Then, we transfected pFLAG-NS2B-3, or pFLAG-NS2B together with pMYC-NS3 into A549 cells, followed by immunofluorescence assay with the indicated antibodies, to observe the distribution of NS2B or NS3 with AXL. The date showed that both NS2B and NS3 extensively colocalized with AXL (Fig. 3B).
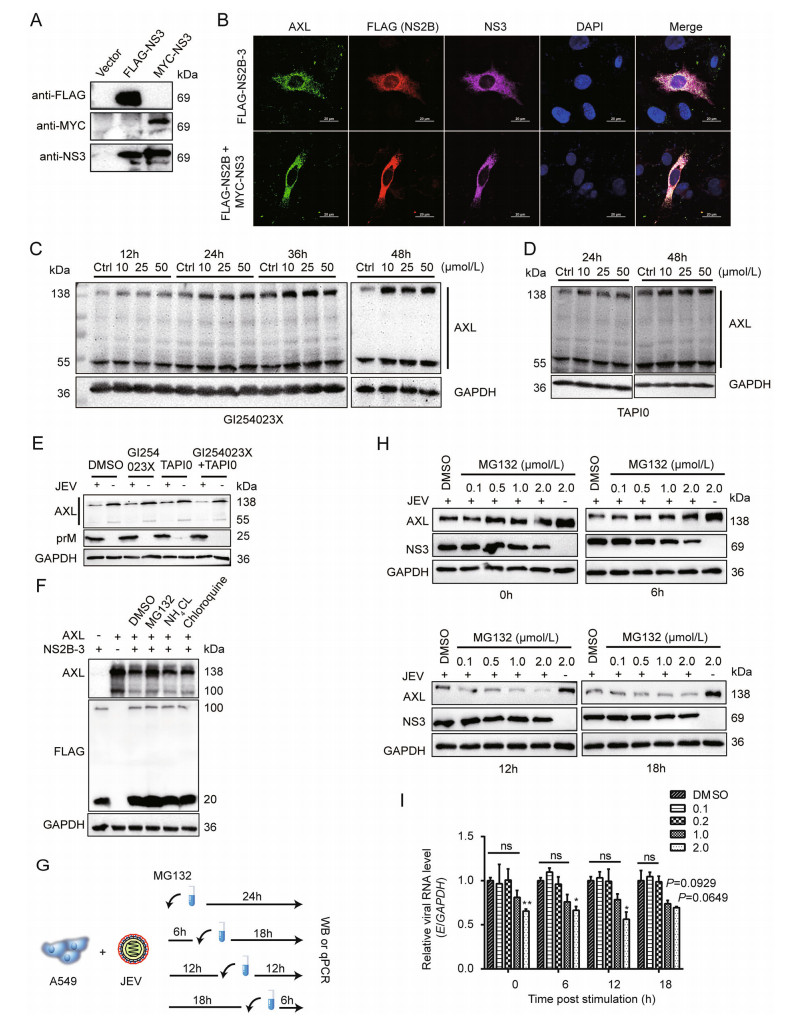
Figure 3. JEV NS2B-3 promotes ubiquitination of AXL for degradation. A pMYC-NS3 was transfected into 293T cells. Anti-MYC antibody was used to test NS3 expression, pFLAG-NS3 served as a control. B pFLAG-NS2B-3 or pFLAG-NS2B and pMYC-NS3 was transfected into A549 cells for 24 h. The cells were captured through confocal microscopy. AXL (green), NS2B or NS2B-3 (red), NS3 or NS2B-3 (purple), nucleus (blue); scale bars, 20 μm. C and D A549 cells were grown in DMEM (2% FBS), and inhibitors at indicated concentration were added. DMSO (50 μmol/L) was used as a control (Ctrl). The cells were lysed at the indicated time period and analyzed by WB. E A549 cells were incubated with JEV (NJ2008, MOI = 1) in DMEM (no FBS) at 37 ℃ for 2 h. After that, they were washed with PBS, and then cultured with fresh DMEM (2% FBS) supplement with indicated inhibitors. At 24 h post infection (hpi), cells were determined by WB. F 293T cells were cotransfected with pAXL and pFLAG-NS2B-3. After 24 h, indicated inhibitors were added and maintained for 12 h. The immunoblot analysis was performed to test AXL protein level. G Schematic representation of MG132 treatment. H and I A549 cells were subject to JEV (NJ2008, MOI = 1) in DMEM (no FBS) for 2 h at 37 ℃. The cells were washed for three times with PBS, and shifted with fresh DMEM. From now on, MG132 or DMSO was added in the indicated time points after JEV infection (0 hpi, 6 hpi, 12 hpi, 18 hpi). After 24 h of infection, total cell lysates were detected by WB (H), JEV E RNA was detected by qRT-PCR (I). Data were expressed as the mean ± SD of three independent experiments, *P ≤ 0.05, **P ≤ 0.01. The results of three independent experiments are shown in the supplementary materials in the form of histograms with bar. WB, Western blotting; SD, standard deviation; ns, not significant; DMSO, dimethyl sulfoxide; JEV, Japanese encephalitis virus; MOI, multiplicity of infection.
Previous studies have reported that the degradation of AXL can be caused by ADAM10 and ADAM17, releasing a soluble 85 kDa extracellular domain (sAXL) and a 55 kDa intracellular domain (Lauter et al. 2019). We examined whether JEV regulated AXL through ADAM10/17 pathway. A549 cells were treated with ADAM10 inhibitor GI254023X or ADAM17 inhibitor TAPI0 at indicated time points. Both inhibitors could prevent the self-degradation of AXL but not JEV-mediated degradation (Fig. 3C–3E, Supplementary Fig. S2G). To explain specific pathways involved in AXL degradation, pAXL and pNS2B-3 were cotransfected into 293T cells in the presence of MG132, NH4Cl, or Chloroquine. Only MG132 could inhibit NS2B-3 mediated AXL degradation (Fig. 3F, Supplementary Fig. S2H), reminding that NS2B-3 degraded AXL through a proteasome pathway. Furthermore, we found the JEV-mediated degradation of AXL was suppressed by MG132, though the presence of MG132 in cell culture medium, which might affect JEV replication at a high dose (2 μmol/L) according to NS3 protein level (Fig. 3G, 3H). At low doses (0.1 μmol/L or 0.5 μmol/L), the replication of JEV was rarely affected by MG132, however, the degradation of AXL mediated by JEV was inhibited significantly compared with dimethyl sulfoxide (DMSO) treated group (Fig. 3H, 3I, Supplementary Fig. S2I).
We next performed coimmunoprecipitation to examine the interaction between AXL and JEV NS2B-3. 293T cells were cotransfected with pFLAG-NS2B-3 and pAXL, followed by treatment with MG132 36 h later. Because NS2B-3 was cleaved in host cells, cell lysate was incubated with anti-FLAG antibody or anti-NS3 antibody. These data indicated that both NS2B and NS3 coimmunoprecipitated with AXL (Fig. 4A). Additionally, we transfected pFLAGNS2B or pFLAG-NS3 individually along with pAXL into 293T cells. The results further indicated that AXL was coimmunoprecipitated with NS2B and NS3 in 293T cells in the present of MG132 (Fig. 4B). Next, to investigate whether JEV NS2B-3 proteins triggered AXL ubiquitination, we cotransfected pAXL, pFLAG-NS2B-3 and pHAubiquitin into 293T cells. The results showed that AXL ubiquitination was markedly increased by JEV NS2B-3, especially in the presence of MG132 (Fig. 4C). In contrast, the individual presence of NS2B or NS3 protein, AXL was rarely ubiquitinated without MG132 treatment. Notably, the AXL ubiquitination level was enhanced after MG132 treatment, and the effect of NS3 was more potent than that of NS2B (Fig. 4D).
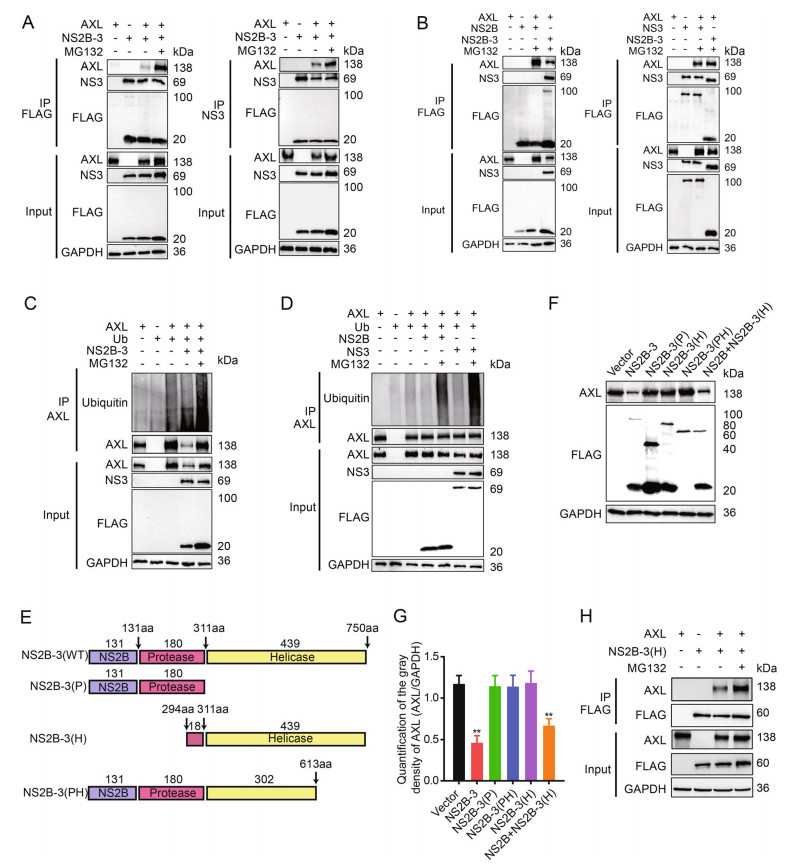
Figure 4. Both NS2B and NS3 are interacted with AXL. A 293T cells were cotransfected with pAXL and pFLAG-NS2B-3 with or without MG132 (10 μmol/L), and immunoprecipitated (IP) with anti-FLAG (left) or anti-NS3 (right). B 293T cells were cotransfected with pFLAG-AXL and pFLAG-NS2B (left) or pFLAG-NS3 (right). pFLAG-NS2B-3 was used as a positive control. IP and immunoblot analysis of AXL, NS2B, NS3, and NS2B-3 were shown. C Coimmunoprecipitation (Co-IP) analysis of AXL ubiquitination in 293T cells cotransfected with pHA-ub and pFALG-NS2B-3. D Co-IP analysis of AXL ubiquitination in 293T cells cotransfected with pHAub and pFLAG-NS2B or pFLAG-NS3. E Schematic representation of NS2B-3 (WT), NS2B-3 (P), NS2B-3 (H), and NS2B-3 (PH). F 293T cells were cotransfected with pAXL and pFLAG-NS2B-3 (WT), pFLAG-NS2B-3 (P), pFLAG-NS2B-3 (PH), or pFLAG-NS2B + pFLAG-NS3 (500 ng + 500 ng) for 36 h. Cells were determined by WB. G Quantification of the gray density of AXL, GAPDH was a loading control. All date were expressed as the mean ± SD of three independent experiments. **P ≤ 0.01. H 293T cells were cotransfected with pAXL and pNS2B-3 (H). After 24 h, MG132 (10 μmol/L) was added and maintained for 12 h. Cell lysates were immunoprecipitated with anti-FLAG antibody. WB, Western blotting; SD, standard deviation.
To further determine the key domains of JEV NS2B-3 responsible for interacting with AXL, we generated a series of JEV NS2B-3 deletion mutants (Fig. 4E) and cotransfected these plasmids along with pAXL into 293T cells. The WB experiments showed only NS2B together with the NS3 helicase domain could degrade AXL, which was consistent with wild-type NS2B-3 (Fig. 4F, 4G). Simultaneously, NS2B-3 (H) (NS3 249 aa–750 aa) interacted with AXL (Fig. 4H).
-
Following activation by ligand, AXL induces tyrosine phosphorylation of PI3K. After activation, the formation of phosphatidylinositol-3, 4, 5-trisphosphate can be used as a second messenger to recruit Akt (Brazil et al. 2004). Activated Akt can phosphorylate various proteins related to the cell death pathway. To verify whether the degradation of AXL by NS2B-3 could lead to cell apoptosis, we transfected pFLAG-NS2B, pFLAG-NS3, or pFLAGNS2B-3 into A549 cells. The results of TUNEL staining and flow cytometry indicated that JEV NS2B-3 promoted A549 cells apoptosis at 48 h post transfection (Fig. 5A, 5B, Supplementary Fig. S2J). Besides, JEV NS3 also promoted cell apoptosis moderately (Fig. 5A, 5B, Supplementary Fig. S2J), which was consistent with previous studies (Yang et al. 2009). Next, to investigate whether NS2B-3 promoted apoptosis via PI3K-Akt cascade, we transfected pFLAG-NS2B, pFLAG-NS3, or pFLAG-NS2B-3 into A549 cells and examined the expression of PI3K-p85 and pAkt (Ser473). WB analysis showed that the expression of PI3K and Akt in pFLAG-NS2B-3 transfected cells was increased, whereas the expression of PI3K-p85 and pAkt (Ser473) was decreased (Fig. 5C, Supplementary Fig. S2K). Next, we cotransfected pAXL or/and pFLAGNS2B-3 into 293T cells, and observed that the phosphorylation of PI3K and Akt was increased by AXL, but blocked by NS2B-3 (Fig. 5D, Supplementary Fig. S2L). We also found that the inhibition of PI3K/Akt phosphorylation caused by JEV was more significant in A549 cells treated with AXL specific siRNA (Fig. 5E, Supplementary Fig. S2M). The association between Gas6/AXL and PI3K/ Akt activation is well known in various cell types (Li et al. 2019). Therefore, we investigated whether Gas6 activated the AXL/PI3K/Akt pathway. In line with the previous research, Gas6 enhanced the phosphorylation of PI3K and Akt through activating AXL (Fig. 5F). Bright-field microscopy images of A549 cells showed that Gas6 rescued NS2B-3 caused cell apoptosis (Fig. 5G). Moreover, TUNEL staining and flow cytometry indicated that Gas6 could also promote cell survival under the condition of JEV infection or pFLAG-NS2B-3 transfection (Fig. 5H, 5I, Supplementary Fig. S2N).
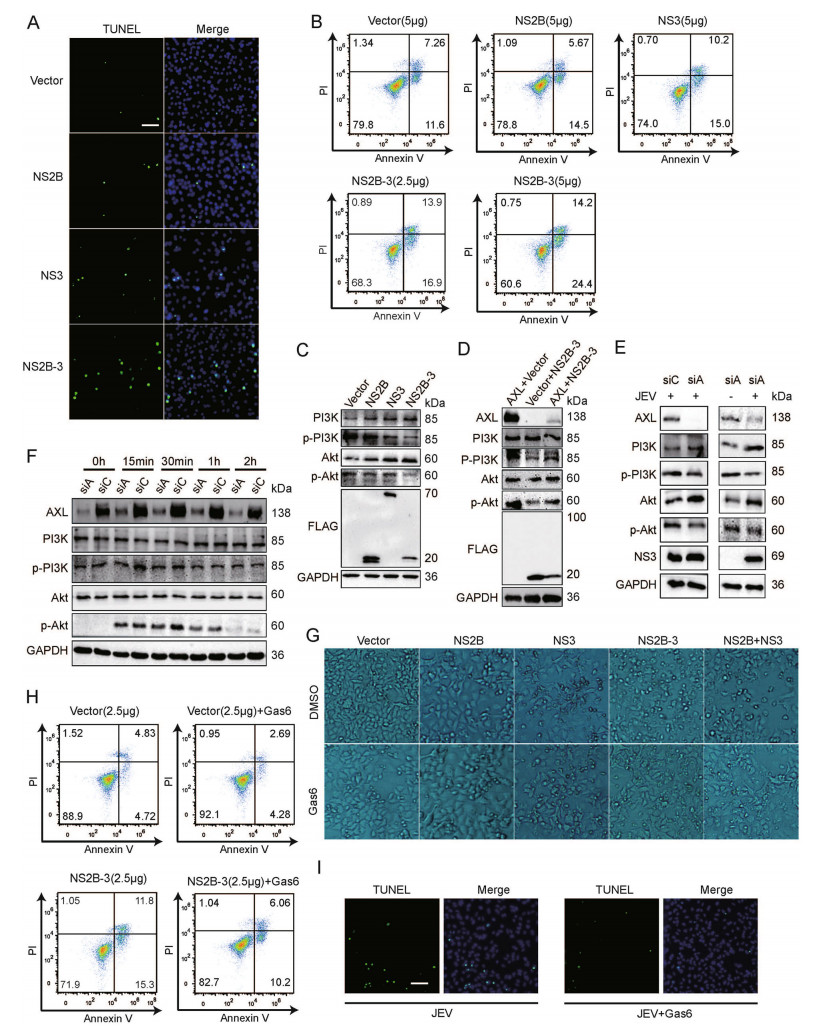
Figure 5. NS2B-3 promotes cell apoptosis in the late stage of JEV infection. A TUNEL staining of pFLAG-NS2B, pFLAG-NS3, or pFLAG-NS2B-3 transfected A549 cells; scale bars, 20 μm. B A549 cells were transfected with plasmids encoding JEV NS2B, NS3, or NS2B-3, and phosphatidylserine (PS) was tested by flow cytometry using Annexin V and Propidium Iodide (PI) binding kit. Experiments performed in duplicate were shown. C A549 cells were transfected with pFLAG-NS2B, pFLAG-NS3, or pFLAG-NS2B-3. 48 h later, cell lysates were analyzed using WB. D 293T cells were cotransfected with pAXL and pFLAG-NS2B-3. After 48 h, WB was performed to measure PI3K and Akt phosphorylation. E A549 cells were transfected by siAXL (siA) or siControl (siC) and challenged with JEV (NJ2008, MOI = 1) for 24 h. WB was performed to measure PI3K and Akt phosphorylation. F A549 cells were transfected by siAXL (siA) and siControl (siC), and then Gas6 (250 ng/mL) was added, the cell lysates were measured by WB at indicated time points. G A549 cells were transfected with pFLAG-NS2B, pFLAG-NS3, pFLAG-NS2B-3, or pFLAG-NS2B + pFLAG-NS3 (500 ng + 500 ng). After 24 h of transfection, cells were treated with Gas6 (250 ng/ mL) for 24 h, and then captured with bright-field microscopy (10 × 10); H A549 cells were transfected with pFLAG-NS2B-3 or vector plasmids in the presence of Gas6 (250 ng/mL), and PS was tested by flow cytometry using Annexin V and PI binding kit. I TUNEL staining of JEV infected A549 cells with or without 250 ng/mL Gas6; scale bars, 20 μm. The results of three independent experiments are shown in the supplementary materials in the form of histograms with bar. WB, Western blotting; JEV, Japanese encephalitis virus; MOI, multiplicity of infection.
-
Except cell survival, previous studies have demonstrated that AXL can enhance various flaviviruses infection, including DENV and ZIKV (Hamel et al. 2015; Savidis et al. 2016). Gas6, the only ligand of AXL, can bind to phosphatidylserine and bridge virions to AXL. The enveloped viruses utilize AXL as an entry receptor, as well as an attenuator of innate immune responses in host cells (Bhattacharyya et al. 2013; Morizono et al. 2011). To explore whether AXL had an impact on JEV replication, we chose A549 cells, which highly expressed AXL, as the model of JEV infection. siA was used to silence endogenous AXL expression in A549 cells (Fig. 6A, 6B, left panels). AXL knockdown showed negligible effects on JEV binding and internalization compared to control siRNA (siC) treated A549 cells (Fig. 6A, 6B, right panels). 293T cells were transfected with pAXL, pAXL-D9, and an empty plasmid respectively (Fig. 6C, 6D, left panels). Ectopic expression AXL in 293T cells still could not enhance JEV binding and internalization (Fig. 6C, 6D, right panels). In addition, to test whether AXL was required for productive JEV infection, we infected AXLknockdown A549 cells with JEV and harvested cells lysates at indicated time points post-infection. Unexpectedly, AXL was unable to enhance JEV infection in A549 cells at different time points (Fig. 6E, Supplementary Fig. S3A). Under the condition of serum-starvation, most cells can secrete Gas6, which can in turn activate AXL in an autocrine manner, and Gas6 is present in the cell medium containing 2% (v/v) FBS (Zizzo et al. 2012). Therefore, we did not artificially add Gas6 in the above experiments. To avoid incorrect results caused by insufficient Gas6, we added sufficient Gas6 (250 ng/mL) to the mediums when JEV infected A549 cells. The concentration of Gas6 in the serum of healthy people ranges from 13 to 100 ng/mL (Morizono et al. 2011), and 250 ng/mL Gas6 is sufficient for various viruses to target AXL for infection (Morizono et al. 2011; Richard et al. 2017). However, the existence of Gas6 still did not promote the infection of JEV in A549 cells (Fig. 6F, Supplementary Fig. S3B). Likewise, we did the same experiences in HeLa cells. AXL could not enhance JEV infection in HeLa cells in either the absence or presence of exogenous Gas6 (Fig. 6G, 6H, Supplementary Fig. S3C, S3D). Compared with WT-293T cells, ectopic expression of AXL in 293T cells still had no effect on JEV infection (Fig. 6I, Supplementary Fig. S3E). These data indicated that AXL did not mediate productive infection of JEV in host cells.
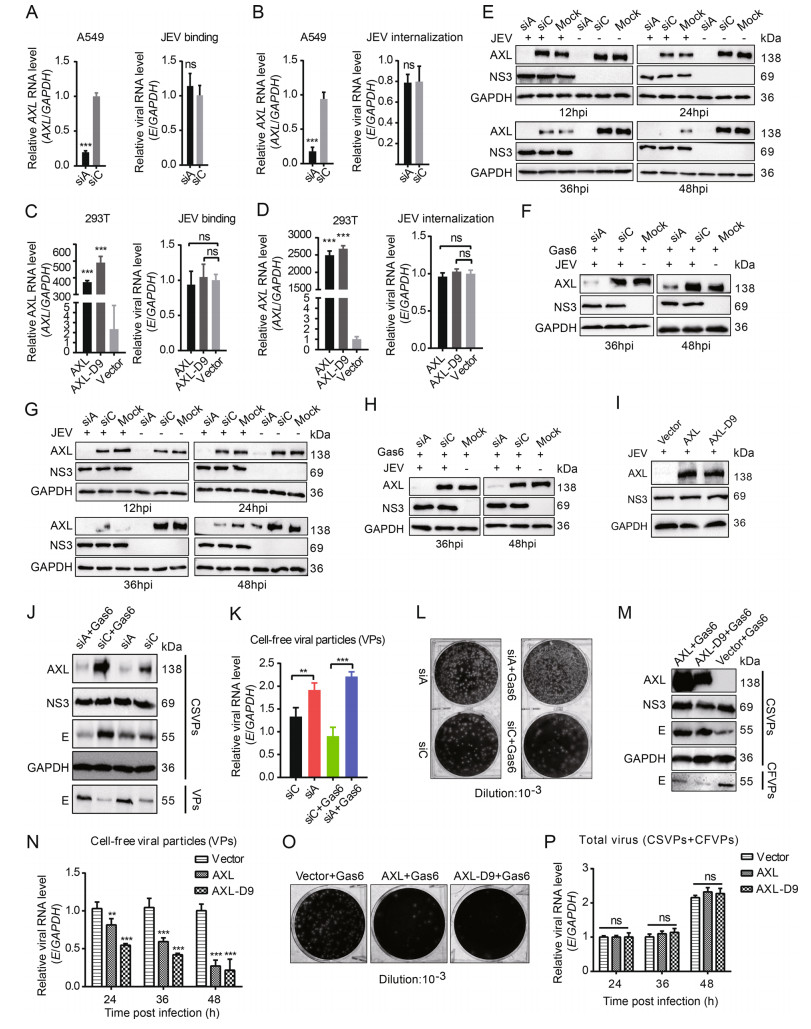
Figure 6. AXL inhibits JEV release. A and C AXL-knockdown A549 cells (A) or ectopic AXL/AXL-D9 expression 293T cells (C) were subject to JEV (NJ2008, MOI = 10) for 1 h at 4 ℃, and detected by qRT-PCR. B and D AXL-knockdown A549 cells (B) or ectopic AXL/ AXL-D9 expressing 293T cells (D) were subject to JEV (NJ2008, MOI = 10) for 1 h at 4 ℃ and washed with PBS to remove unbound viral particles. Cells were transferred to 37 ℃ for 30 min to allow virus entry and then treated with proteinase K. The viral RNA level was determined by qRT-PCR. E and G AXL-knockdown A549 (E) or HeLa (G) cells were subject to JEV (NJ2008, MOI = 1), and the cell lysates were collected at indicated time points. F and H AXLknockdown A549 (F) or HeLa (H) cells were challenged with JEV (NJ2008, MOI = 1) with 250 ng/mL Gas6. After indicated time points of viral infection, the cell lysates were analyzed by WB. I 293T cells expressing AXL or AXL-D9 were incubated with JEV (NJ2008, MOI = 1). After 24 h of infection, JEV NS3 protein was analyzed by WB. J AXL-knockdown A549 cells were infected by JEV in the absence or presence of Gas6, MOI = 1, 36 h post infection (hpi). WB was performed to test cell-associated viral particles (CSVPs, intracellular) and cell-free viral particles (CFVPs, supernatant) by using anti-NS3 or anti-E antibodies. K and L Cell-free viral particles were examined by qRT-PCR (K) and plaque assays (L). M 293T cells were transfected with pAXL or pAXL-D9, and then infected by JEV with Gas6 for 36 h, MOI = 1. CSVPs and CFVPs were determined using WB. N CFVPs at 24, 36, 48 hpi were measured by qRT-PCR. O CFVPs at 36 hpi were measured by plaque assays. P AXL or AXLD9 expressing 293T cells were incubated with JEV (NJ2008, MOI = 1). The total RNA level of E was measured by qRT-PCR at indicated time points. All qPCR date were expressed as the mean ± SD of three independent experiments. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001. The results of three independent experiments are shown in the supplementary materials in the form of histograms with bar. WB, Western blotting; SD, standard deviation; ns, not significant. JEV, Japanese encephalitis virus; MOI, multiplicity of infection.
However, at 36 h post infection, we found Gas6 might inhibit the degradation of AXL by JEV (Fig. 6E–6H). This is an interesting phenomenon. Without exogenous addition, Gas6 expressed by A549 cells was not enough to inhibit the degradation of AXL by JEV in the late stage of infection (Fig. 6E, 6G). Consistent with the above results (Fig. 6E), down-regulating the expression of AXL in A549 cells did not affect JEV replication, according to NS3 protein level detected in cell lysates. However, the virus particles in cell lysates were obviously decreased based on E protein (Fig. 6J). Intriguingly, an evident increase of the viral particles in the supernatant were detected in siA-treated groups compared with that in siC-treated groups, especially in the presence of exogenous Gas6 (Fig. 6J). Consistently, the qRT-PCR and plaque assays results showed that more virus particles released into the supernatant in siA-treated groups (Fig. 6K, 6L). To further investigate the effect of AXL on JEV release in the presence of Gas6, we ectopically expressed AXL in 293T cells and then inoculated with JEV. Ectopic AXL expression in 293T cells decreased the amount of virus particles in the supernatant (Fig. 6M– 6O). Accordingly, more JEV E proteins were detected in cell lysates. In general, there was no significant difference of total JEV production in 293T cells transiently expressing AXL compared to control 293T cells (Fig. 6P). These results indicated that AXL did not affect the proliferation of JEV, but changed the distribution of JEV, resulting in fewer virions release to the supernatant.
JEV Infection Down-Regulates AXL Protein Expression
JEV NS2B-3 Protein is Crucial for DownRegulating AXL Expression
JEV NS2B-3 Degrades AXL through the Proteasome Pathway
NS2B-3 Promotes Cell Apoptosis through Inhibiting the AXL/PI3K/Akt Pathway
Down-Regulating the Expression of AXL in Cells Promotes Virus Particles Release
-
Our results clarified that AXL had no significant effect on the infection process of JEV at the early stage, including virus binding, internalization, and proliferation. However, in the late stage of JEV infection, AXL increased more virus particles retained in viral-producing cells, while reduced the viral particle release to the supernatant. Besides, we also found that JEV NS2B-3 caused AXL proteasome-dependent degradation. The supplement of AXL ligand Gas6 could inhibit the degradation of AXL by JEV, thereby blocking the release of the virus.
Several studies have suggested that AXL is involved in the infection of flavivirus. For example, AXL can promote DENV entry into host cells via the clathrin-dependent endocytosis pathway (Meertens et al. 2012). ZIKV hijacks AXL to antagonize type I interferon signaling via increasing SOCS1 expression (Chen et al. 2018). However, other studies have also provided conflicting results. There is no difference in susceptibility to ZIKV between AXL-/- mice (AXL gene-deficient mice) and WT mice (Hastings et al. 2017). Depletion of AXL failed to protect human neural progenitor cells and brain organoids from ZIKV infection (Wells et al. 2016). These results further illustrated that AXL played complicated roles in flavivirus infection. A recent study found that AXL promoted the survival of JEV-infected macrophages, and reduced the neuroinvasion of JEV (Wang et al. 2020). Our experiment also proved that JEV NS2B-3 inhibited the activation of the PI3K/Akt cascade by degrading AXL, thereby leading to cell apoptosis. Meanwhile, the loss of AXL can also cause apoptosis, which is not mediated by caspase-3 (Wang et al. 2020). However, the pathogenesis of West Nile virus (WNV)-induced encephalitis is caused by neuronal apoptosis mediated by caspase-3 (Samuel et al. 2007). Cell death may be a potential mechanism for cells to resist infection. The host cells can limit the spread of the virus by destroying the infected cells (Okamoto et al. 2017). In addition, the virus can promote the formation of progeny virus particles and the release of cell contents through the death of cells, thereby causing a violent inflammatory response and leading to the spread of the virus (Martins Sde et al. 2012; Servet-Delprat et al. 2000). In terms of cell death, flaviviruses have some commonalities. During virus infection, how to balance cell survival and death is still a core issue that needs to be solved urgently.
Our results proved that JEV NS2B-3 triggered AXL ubiquitination and degradation through the proteasome pathway. As the protein complex of flavivirus, NS2B-3 has been reported to bind to a variety of host proteins to promote virus replication (Lennemann and Coyne 2017; Rodriguez-Madoz et al. 2010). Most of the research has focused on the interactions between NS2B-3 and innate immune protein molecules (Aguirre et al. 2012; Wu et al. 2017). The helicase fragment of ZIKV NS3 could trigger mitochondrial antiviral signaling protein (MAVS) ubiquitination to circumvent host innate immunity (Li et al. 2019). In fact, we also found that the helicase fragment of JEV NS3 could interact with AXL. While AXL can be used as a natural immunosuppressant (Lemke 2013), degradation by JEV NS2B-3 does not seem to be conducive to virus self-replication. Our research also found that AXL inhibited the release of virus particles. Like AXL, TIM-1 is also a member of the PS receptors family. According to reports, TIM-1 could inhibit the release of HIV, but the event is antagonized by HIV Nef protein (Li et al. 2014, 2019). Previous study also showed that AXL could restrict the release of Ebola virus (EBOV) (Li et al. 2014). However, unlike flaviviruses assembling in the endoplasmic reticulum, EBOV assembles in cell membrane and buds from the host cell (Gordon et al. 2019). EBOV and JEV are both enveloped viruses, and their surface exposed PS may bind to AXL through Gas6. This also explains why the addition of Gas6 can promote AXL-mediated suppression of virus release. Furthermore, clusters of enteroviruses are packaged within PS lipid-enriched vesicles that are non-lytically released from cells (Chen et al. 2015). The rich PS on surface of the vesicles may be bound by Gas6/AXL, thereby blocking the release of clusters of viral particles. Although up to now, few studies believe that mosquito-borne flaviviruses are associated with host cells through vesicles (Caobi et al. 2020). However, hepatitis C virus (HCV) is known to incorporate genomic RNA into exosomes (Anderson et al. 2016). Another tick-transmitted flavivirus, Langat virus (LGTV), uses exosomes for tick-tomammalian cross-host transmission. Through the mouse model, LGTV caused widespread dissemination in neurons through exosomes, leading to severe encephalitis (Zhou et al. 2018). In addition, exosomes facilitate the transmission of ZIKV across neurons by functioning as mediators, enhancing neuropathology of ZIKV (Zhou et al. 2019). Whether flaviviruses are released in the form of phosphatidylserine vesicles and are intercepted by AXL is not known, but this is definite a fascinating question.
We also found that Gas6 inhibited the degradation of AXL by JEV. We speculated that the addition of Gas6 caused AXL autophosphorylation, ubiquitination, or dimerization. The modification and conformational changes of AXL make JEV unable to degrade it. These issues need more in-depth research. The specific degradation of AXL by JEV to release virus particles may have significant implications for virus transmission and spread in vivo. In particular, the primary mouse peritoneal macrophages that highly express AXL are susceptible to JEV infection (Lemke and Rothlin 2008). After 24 h of infection with JEV, AXL was almost undetectable in macrophages. Macrophages are one of the main target cells while JEV infects cells locally in the skin surrounding the mosquito bite (Filgueira and Lannes 2019). The rapid degradation of AXL mediated by JEV in macrophages may lead to the release of a concentrated bolus of viral particle around the vicinity of uninfected cells, and locally enhance the spread of JEV.
-
This work was carried out with support of grants from the National Key Research and Development Plan of China (Grant No. 2016YFD0500402), the National Natural Science Foundation of China (Grant No. 31772756), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
-
SDX and RBC designed the experiments. SDX, ZJL, XMY, and TTL carried out the experiments. SDX and RBC analyzed the data. JHP and YD provided constructive suggestions. SDX and RBC wrote the paper. SDX and RBC checked and finalized the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
The isolation experiments of primary peritoneal macrophages cells were conducted under the guidelines of the regional Animal Ethics Committee and the rules for experimental animals of Nanjing Agricultural University.















 DownLoad:
DownLoad: