HTML
-
Porcine reproductive and respiratory syndrome (PRRS) is characterized by reproductive failure in sows, severe respiratory disease and poor growth performance in piglets and growing pigs, and causes significant economic loss to the swine industry worldwide (Chand et al. 2012). PRRS is caused by PRRSV, an enveloped, single-stranded positivesense RNA virus that clusters in the order of Nidovirales and the family of Arteriviridae (Kuhn et al. 2016). The genome of PRRSV is proximately 15 kb in length and contains over 10 open reading frames (ORFs). PRRSV is divided into the European genotype (type Ⅰ) and the North American genotype (type Ⅱ). Although the overall disease phenotype and clinical symptoms are similar, the nucleotide identification of these two genotypes accounts for approximately only 60% (Mardassi et al. 1994). Since it was first reported in 1987 in the United States, PRRS has been reported in almost all pork-producing countries, with particularly high severity in China (Li et al. 2007). In 2013, the annual loss caused by PRRS to the American swine industry was approximately 664 million USD.
Currently, vaccination is the most common method of controlling PRRSV infections (Han et al. 2017). However, vaccines can only provide protection against isotypical strain infections and have very limited efficacy on newly emerging mutant strains. PRRSV has the intrinsic ability to frequently adapt and evolve to form new strains due to its high mutation rate at 3.29 × 10-3 substitutions per nucleotide site per year (Tian et al. 2007). In addition, clinical protective effects of PRRSV vaccines are limited by the induction of antibody-dependent enhancement (ADE) (Cancel-Tirado et al. 2004). Therefore, there is an urgent need to develop novel antiviral measures against PRRSV infections.
Previous studies have shown that some natural compounds have antiviral activities against PRRSV infections, including glycyrrhizin (Duan et al. 2015), morpholino oligomer (Opriessnig et al. 2011), proanthocyanidin A2 (Zhang et al. 2018b), xanthohumol (Liu et al. 2019), and platycodin D (Zhang et al. 2018a). Despite these findings, no effective drugs against PRRSV infection are commercially available.
Andrographis paniculate nees (Burm. F), a member of the Acanthaceae family, is a medicinal plant that has long been used in China, India, Japan, Korea, and other Asian countries to treat a range of illnesses, including influenza, fever, sore throat, acute or chronic cough, laryngitis, colitis, urinary infection, and diarrhea (Cáceres et al. 1999). Andrographolide (Andro) is the most abundant diterpene lactone in the leaves and stems of A. paniculata. Over the last 40 years, Andro and three of its derivatives, "Chuanhuning" (14-dehydroxy-11, 12-didehydroandrographolide-3, 19-bis potassium salt), "Yanhuning" (14-dehydroxy-11, 12-didehydroandro-grapholide-3, 19-bis potassium sodium salt), and "Lianbizhi" (14-dehydroxyandrographolide-12-sulfonic acid sodium salt), have been used clinically in China to treat bacterial and viral infections (Luo et al. 2017; Duan et al. 2020). These drugs are effective in relieving the symptoms of inflammation, fever, and pain induced by bacterial and viral infections (Puri et al. 1993). In addition, Andro and its derivatives were demonstrated to have a wide range of effects against virus infections, including human immunodeficiency virus (HIV) (Calabrese et al. 2000), herpes simplex virus (HSV) (Wiart et al. 2005), hepatitis C virus (HCV) (Lee et al. 2014), chikungunya virus (CHIKV) (Wintachai et al. 2015), dengue virus (DENV) (Panraksa et al. 2017), and influenza virus (Chen et al. 2009). Considering the results of these previous studies, we sought to determine whether Andro and its clinical derivatives have anti-PRRSV activities.
In the present study, we evaluated the anti-PRRSV activity of Andro and its clinical derivatives in Marc-145 and PAM cells. Our results demonstrated that Andro and its derivative potassium dehydrographolide succinate (PDS, also named "Chuanhuning") exhibited significant antiviral activity in Marc-145 and PAM cells against various PRRSV strains infection with minimal cytotoxicity. Furthermore, we demonstrated that Andro and PDS inhibited PRRSV replication via differential mechanisms of action. Since Andro and PDS have been extensively used as anti-inflammatory drugs, our work provides interesting insight into their potential applications in controlling PRRS.
-
Marc-145 cells, a PRRSV-permissive cell line derived from the African green monkey kidney cell line MA-104, were obtained from the American Type Culture Collection (ATCC). Marc-145 cells were grown in Dulbecco's minimum essential medium (DMEM; Gibco, UT, USA) supplemented with 10% fetal bovine serum (FBS; Biological Industries, Kibbutz Beit Haemek, Israel), 100 IU/mL penicillin, and 100 lg/mL streptomycin at 37 ℃ with 5% CO2. Porcine alveolar macrophages (PAMs) were obtained from the lungs of 4- to 6-week-old PRRSV-negative LargeWhite piglets (Xinli Pig Farm, Wuzhou, China) by lung lavage, according to a previously described method (Ait-Ali et al. 2007). Briefly, the lungs were washed three times with pre-cooled phosphate buffered saline (PBS) solution containing penicillin (300 IU/mL) and streptomycin (300 lg/mL). The cells were centrifuged at 800 × g for 10 min, resuspended in RPMI-1640 (Gibco) supplemented with 10% FBS, 100 IU/mL penicillin, and 100 lg/mL streptomycin at 1 × 106 cells/mL in a 6-well plate, and then incubated at 37 ℃ for 2 h. The non-adherent cells were removed and the adherent cells (PAMs) were collected. Three type 2 PRRSV strains, GD-HD (GenBank no. KP793736), XH-GD (GenBank no. EU624117), and NADC30-like HNhx (GenBank no. KX766379) strains (Xie et al. 2013), were propagated in Marc-145 cells in DMEM with 3% FBS (essential medium). Virus preparations were stored at - 80 ℃. Virus titers were determined using a microtitration infectivity assay.
Briefly, virus preparations were tenfold serially diluted in essential medium. Confluent monolayers of Marc-145 cells or PAMs prepared in 96-well plates were inoculated in quadruplicate with 100 μL of each sample and incubated for 2 h at 37 ℃. The inoculum was then discarded, and the cell monolayer replenished with fresh essential medium, incubated for an additional 72 h, and monitored for cytopathic effects (CPE) daily. The titer of each preparation was calculated based on the amount of CPE and was expressed as a 50% tissue culture infective dose (TCID50)/mL.
-
Andro and PDS were purchased from Chendu Pufei De Biotech Co., Ltd. (Chendu, China), with a purity of C 99.3%. Ribavirin, a broad-spectrum antiviral agent, was purchased form Star Lake Bioscience Co., Ltd. (Zhaoqing, China). Andro and ribavirin were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, MA, USA), and PDS was dissolved in normal saline and diluted with essential medium before use. The final concentration of DMSO in the culture medium was < 0.4%.
-
The cytotoxicity of Andro and PDS was evaluated using an MTT assay (Yang et al. 2013). Briefly, for Marc-145 cells, 5 × 104 cells (per well) were seeded in 96-well plates and grown at 37 ℃ for 36 h. For PAMs, 2 × 105 cells (per well) were seeded in 96-well plates and incubated at 37 ℃ for 12 h. The medium was replaced with a fresh medium containing serially diluted compounds, and the cells were further incubated for 48 h. The culture medium was removed and replaced with 100 μL 3-(4, 5-dimethylthiozol-2-yl)-3, 5-dipheryl tetrazolium bromide (MTT; Sigma-Aldrich) solution (0.5 mg/mL in PBS) and incubated at 37 ℃ for 4 h. After removal of the supernatant, 150 μL of DMSO was added to all wells for 10 min at 37 ℃ to dissolve the formazan crystals. Cell viability was measured as the absorbance at 490 nm with a microplate reader (Thermo Fisher scientific, MA, USA) and was expressed as a percentage of the control level. The mean optical density (OD) values taken from six wells per treatment were used as the cell viability indexes. The 50% cytotoxic concentration (CC50) was analyzed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
-
Marc-145 or PAM cell monolayers grown in 96-well plates were infected with PRRSV (0.05 MOI for Marc-145 cells, 1 MOI for PAMs) in essential medium for 2 h at 37 ℃. Supernatants were removed and fresh DMEM containing different concentrations of each compound were added. Cells and supernatants were then collected at the indicated time points post-infection and subjected to three freeze–thaw cycles at - 80 ℃ and 4 ℃, respectively, to ensure maximal release of cellular virions. The final supernatant viral titer was determined by the end point dilution assay using Marc-145 cells and was expressed as log10 TCID50/mL (Ma et al. 2002).
-
For immunostaining, the PRRSV-infected or uninfected cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.25% Triton X-100 for 10 min at room temperature (RT), blocked with 1% bovine serum albumin (BSA) for 60 min at RT, and then incubated with a mouse monoclonal antibody against the N-protein of PRRSV (clone 4A5, 1:400 dilution; MEDIAN Diagnostics, Korea) at 4 ℃ overnight. After three washes with PBS, the cells were incubated for 1 h at RT with a goat anti-mouse secondary antibody conjugated with Alexa Fluor® 568 (red) (Cell Signaling Technology, MA, USA) at a 1:1000 dilution. Nuclei were counterstained with 50 μL of 4, 6-diamidino-2-phenylindole (DAPI, 300 nM; SigmaAldrich) (blue). Immunofluorescence was captured using the Leica DMI 4000B fluorescence microscope (Leica, Wetzlar, Germany).
The relative N-protein level (%) of each image was calculated based on the fluorescence OD using Image J software. The results from compound-treated samples were compared to those from corresponding DMSO-treated control groups (set as 100%). The EC50 value (the concentration required to protect 50% cells from PRRSV infection) was determined by plotting the relative N-protein level as a function of compound concentration and was calculated using GraphPad Prism 7.0 software.
-
Marc-145 cells grown on 10-mm cover slips (MatTek, MA, USA) were infected with PRRSV (0.05 MOI) for 2 h. The cells were washed three times with PBS and then incubated with fresh medium containing 45 μmol/L of Andro or 560 μmol/L of PDS. At 24 h post infection, cells were fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and blocked with 1% BSA following the IFA protocol as described above. The cells were then incubated with a rabbit monoclonal antibody against the NF-κB p65 (1:1000; CST, MA, USA) at 4 ℃ overnight. After three washes with PBS, the cells were incubated for 1 h at RT with a goat anti-rabbit secondary antibody conjugated with Alexa Fluor® 488 (green) (1:1000; CST, MA, USA). Nuclei were counterstained with 50 μL of 4, 6-diamidino-2-phenylindole (DAPI, 300 nmol/L; SigmaAldrich) (blue). Immunofluorescence was imaged using a TCS SP8 confocal laser scanning microscope (Leica) (Luo et al. 2018).
-
Total RNA was extracted from the cells or culture supernatants using the total RNA rapid extraction kit (Fastagen, Shanghai, China) following the manufacturer's instructions. RNA was reverse-transcribed into first-strand cDNA using a reverse transcription kit (TaKaRa, Dalian, China). PCR amplification was performed on 1 μL of reversetranscribed product with primers designed against PRRSVNSP9, cytokines (TNF-α, IL-6, and IL-1β), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; used as the endogenous control). The primers used for PCR amplification are listed in Supplementary Table S1. Real-time PCR was performed using 2 × RealStar Green Power Mixture (containing SYBR Green I Dye) (Genstar, Beijing, China) on the CFX96 Real-time PCR system (Bio-Rad, CA, USA). The relative mRNA expression was calculated by the 2-ΔΔCT method using DMSO-treated infected cells or DMSO-treated mock-infected cells as reference samples for determining PRRSV-NSP9 and cytokine gene expression, respectively (Schmittgen and Livak 2008). To assess the effect of Andro and PDS on transcriptional activation of cytokines in PRRSV-infected cells, the relative fold change of the gene expression of each cytokine was calculated and compared between virus-infected and mockinfected PAM cells and between Andro and PDS-treated virus-infected and virus-infected cells.
-
PRRSV-infected or uninfected Marc-145 cells treated with Andro and PDS were lysed in RIPA lysis buffer containing 1 mmol/L phenylmethylsulfonylfluoride (Beyotime, Haimen, China) at 4 ℃. The supernatant was harvested after centrifugation (15, 000 × g for 10 min at 4 ℃), and the total protein content of each sample was measured using the BCA protein assay kit (Beyotime). Total protein (10 lg per sample) was electrophoresed onto a 12% SDS-PAGE gel and transferred to polyvinylidene-fluoride (PVDF) membranes (Millipore, MA, USA). After blocking, the membranes were incubated with a mouse anti-PRRSV N-protein monoclonal antibody (clone 4A5), an antibody against a-Tubulin (1:1000; Beyotime), or antibodies against NF-κB p65, Phospho-NF-κB p65 (Ser536) (p-p65), IκBα, and Phospho-IκBα (Ser32) (p-IκBα) (1:1000; CST, MA, USA). Membranes were incubated with HRP-conjugated goat anti-mouse or anti-rabbit IgG (H–L) secondary antibodies (1:3000; Beyotime). The Odyssey system (LICOR, CT, USA) was used to analyze the protein bands on PVDF membranes.
-
A time-of-addition assay was performed as previously described (Zhang et al. 2018a) with some modifications. Briefly, Marc-145 cells were grown in 24-well plates to confluence and then infected with the PRRSV GD-HD strain at 0.05 MOI for 2 h at 37 ℃. Andro and PDS were added before (pre-treatment), during (co-treatment), or after (post-treatment) PRRSV infection. For pre-treatment, cells were incubated with Andro and PDS for 2, 4, 6, or 8 h at 37 ℃, followed by three washes with PBS, and then infected with PRRSV for 2 h. For co-treatment, the cells were simultaneously incubated with PRRSV at 37 ℃. After 2 h, the mixture was removed, and the cells were washed three times with PBS before adding fresh medium. For post-treatment, the cells were first infected with PRRSV for 2 h at 37 ℃, and then incubated in the fresh medium containing Andro and PDS for 2, 4, 6, or 8 h, followed by continuous incubation in fresh medium. At 24 h post infection (hpi), the samples were submitted to IFA to determine the PRRSV-infected cell count.
-
To investigate whether Andro and PDS directly interact with the virus, 100 μL of PRRSV (0.05 MOI) was mixed with various concentrations of Andro or PDS in essential medium (0.9 mL) and incubated for 1 h at 37 ℃. Then, PRRSV and Andro or PDS were separated using ultrafiltration centrifugation. Briefly, the mixture of PRRSV and Andro or PDS was added to an ultrafiltration tube (0.5 mL, 30 K cutoff) and centrifuged at 7500 × g for 10 min at 4 ℃. PRRSV trapped in the ultrafiltration tube was washed twice with essential medium to remove residual Andro and PDS and was then resuspended in essential medium for infecting Marc-145 cells grown in 6-well plates for 2 h. After three washes with PBS, the cells were cultured in fresh medium for an additional 48 h at 37 ℃.
-
PRRSV GD-HD stock (26 mL; 1 × 106 TCID50/mL) was concentrated by centrifugation at 160, 000 × g at 4 ℃ for 4 h in a 70Ti rotor (Beckman Coulter, USA). The precipitate was dissolved in 0.9% NaCl (500 μL). The virus diluent was treated with various concentrations of PDS at 37 ℃ for 1 h, and then absorbed onto formvar/carboncoated 200-mesh copper grids and negatively stained with 2% phosphotungstic acid. After air-drying, the sample grid was examined under a transmission electron microscope (Talos L120C; Thermo Fisher Scientific, USA) operating at 100 kV.
-
The cellular level of reactive oxygen species was determined using a dichlorofuorescein (DCF) ROS assay kit according to the manufacturer's instructions (Beyotime). Marc-145 cells were infected with PRRSV (0.05 MOI) for 2 h and then treated with 45 μmol/L Andro or 560 μmol/L PDS for a further 24 h. Subsequently, 2, 7-dichlorodi-hydrofluorescein diacetate (DCFH-DA) (10 mmol/L) was added to the cell culture and incubated for 30 min at 37 ℃. Then, Marc-145 cells were washed and imaged using the Leica DMI 4000B fluorescence microscope (Leica), with an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Flow cytometry, as described by Hung (Hung et al. 2021), was used to determine the cellular ROS level. Briefly, the cells were treated as described above. Then, the cells were digested with trypsin, centrifuged, and resuspended in FACS buffer. Fluorescence intensity was measured by Cytoflex (Beckman Coulter), and the data were analyzed using CyExpert (Beckman Coulter).
-
The cells were infected with PRRSV or stimulated with H2O2, and then treated with Andro or PDS as described above. After three-time washes, cells were lysed by RIPA lysis buffer. The lysate was collected and centrifuged at 15, 000 × g for 10 min at 4 ℃. The protein concentration of the supernatant was determined using the BCA detection kit (Beyotime). The levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) in the cells were detected using an SOD detection kit, a GSH detection kit, and a trace MDA detection kit (Beyotime), respectively, according to the manufacturer's instructions.
-
All experiments were performed at least three times. The results are presented as mean ± standard deviation (SD). Statistical significance was determined using Student's t-test when only two groups were compared and using oneway analysis of variance (ANOVA) when more than two groups were compared. With regard to statistical significance, *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, and ###P < 0.001 were considered to be statistically significant at different levels.
Cell Lines and Viruses
Preparation of Andro and PDS
Cytotoxicity Assay
Viral Titer Titration
Indirect Immunofluorescence Assay (IFA)
Laser Scanning Confocal
Quantitative Real-Time PCR (qRT-PCR)
Western Blot Analysis
Time-of-Addition Assay
Direct PRRSV-Andro/PDS Interaction
Transmission Electron Microscopy Analysis
Determination of Reactive Oxygen Species (ROS)
Analysis of Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px), and Malondialdehyde (MDA)
Statistical Analysis
-
The chemical structures of andrographolide (Andro) and dehydroandrographolide potassium succinate (PDS) are shown in Fig. 1A. The cytotoxicity of Andro and PDS was first measured using the MTT assay, and their CC50 values were calculated to be 126.8 μmol/L and 29, 409 μmol/L (Fig. 1B), respectively, indicating that Andro has minimal cytotoxicity and PDS has no cytotoxicity on Marc-145 cells. The inhibitory effects of Andro and PDS on three different PRRSV strains (GD-HD, XH-GD, and NADC30-like) were assessed by IFA. As shown in Fig. 1C and 1D, replication of PRRSV was significantly inhibited by both Andro and PDS in a dose-dependent manner. The EC50 values of Andro against three tested PRRSV strain infections ranged from 11.75 to 15.29 μmol/L, while the EC50 values of PDS ranged from 57.15 to 85.41 μmol/L, as determined by analyzing the cell infection rate from IFA images. Ribavirin, a well-known inhibitor of viral RNA polymerase, was used as a positive antiviral drug control in this study. Our results show that 160 μmol/L of ribavirin exhibited significant inhibition on the three tested PRRSV strains in Marc-145 cells.
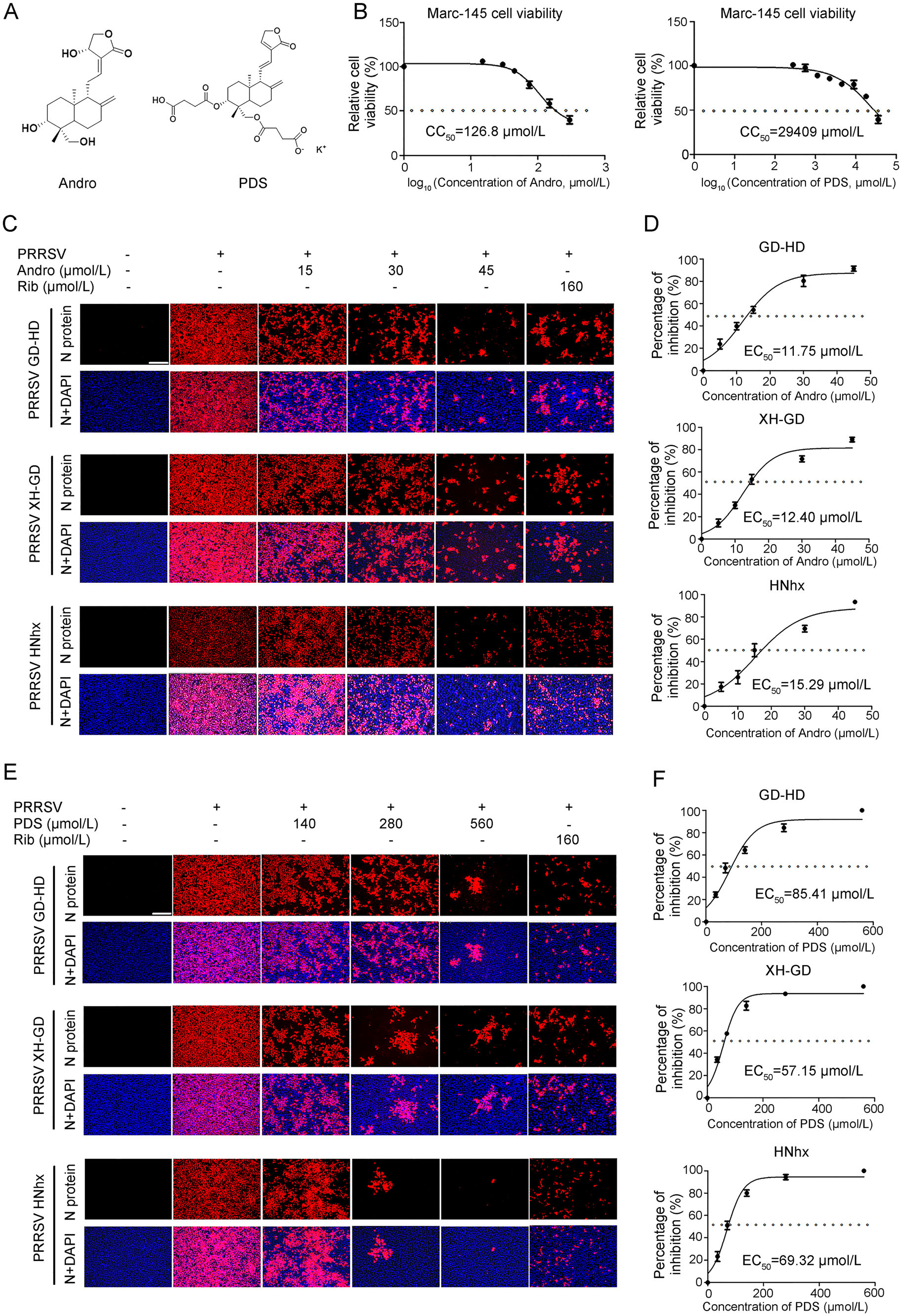
Figure 1. Andro and PDS inhibit PRRSV replication with minimal cytotoxicity in Marc-145 cells. (A) Chemical structures of andrographolide (Andro) and potassium dehydroandrographolide succinate (PDS). (B) Cellular toxicity of Andro and PDS was examined in Marc-145 cells using an MTT assay. (C and D) Antiviral activity of Andro and PDS against PRRSVs (GD-HD, XH-GD, and NADC30- like HNhx strains) infection in Marc-145 cells was examined using indirect immunofluorescence assay (IFA). Cells grown in 96-well plates were infected with PRRSV (0.05 MOI) for 2 h at 37 ℃ and then cultured in fresh medium containing various concentrations of Andro or PDS. The IFA for the N protein of PRRSV was performed at 48 hpi using Alexa Fluor 568-conjugated goat anti-mouse secondary antibody (red). Nuclei were counterstained with 4, 6-diamidino-2- phenylindole (DAPI) (blue). Representative IFA images of three independent experiments are shown in C and D. D and F demonstrate the percentage of inhibition based on the fluorescence optical densities (OD) of the images from three independent experiments. Image J was used to digitize image OD. Scale bar: 250 μm.
To evaluate whether Andro and PDS have sustaining inhibition effect on PRRSV replication, we further analyzed the kinetics of the viral titer, viral RNA, and protein expression from 24 to 72 hpi. As shown in Fig. 2A and 2B, treatment with Andro and PDS resulted in a significant reduction in viral titer in a dose-dependent manner at all indicated time-points. Treatment with 45 μmol/L of Andro or 560 μmol/L of PDS led to a 3.2 log or 6.5 log reduction in progeny virus production at 72 hpi, respectively, compared with the DMSO-treated control. The inhibitory effects of Andro and PDS on PRRSV replication were further confirmed by the reduction in viral NSP9 RNA levels (Fig. 2C and 2D) and protein expression (Fig. 2E and 2F). These results show that Andro and PDS have a sustained inhibitory effect on PRRSV infection.

Figure 2. Confirmation of the anti-PRRSV activity of Andro and PDS in Marc-145 cells. Marc-145 cells were cultured in 12-well plates for 24 h. Cells were infected with 0.05 MOI of PRRSV GD-HD for 2 h and incubated with fresh medium containing different concentrations of Andro or PDS. Samples were collected at 24, 48, and 72 h post infection. The supernatants were used to determine the virus titers using the end-point dilution assay (A and B), and the cells were used for the analysis of relative PRRSV NSP9 mRNA levels by qRT-PCR (C and D) and viral N protein expressions using Western blot (E and F). α-Tubulin was used as a loading control, and DMSO-treated sample (0 μmol/L Andro and PDS) was used as the control. **P < 0.01, and ***P < 0.001 compared to the DMSO-treated control.
-
As PAMs are the major target cells of PRRSV infection in pigs in vivo, we further investigated whether Andro and PDS also inhibit PRRSV replication in PAMs. The CC50 value of Andro and PDS in PAMs was measured by MTT assay and was calculated to be 173.2 μmol/L and 9359 μmol/L, respectively (Fig. 3A). Next, the anti-PRRSV activity of Andro and PDS was evaluated by determining the virus titer, viral N protein expression and mRNA level. Infected PAMs were detected by IFA, while the viral inhibition rate in the presence of Andro or PDS was assessed. PRRSV replication was significantly suppressed at 90 μmol/L Andro and 560 μmol/L PDS, respectively (Fig. 3B). The EC50 of Andro and PDS against GD-HD PRRSV replication in PAMs was 32.98 μmol/L and 47.16 μmol/L, respectively (Fig. 3C). In PAM cells, Andro or PDS treatments also resulted in a significant reduction in virus titers (Fig. 3D), viral RNA levels (Fig. 3E) and N protein levels (Fig. 3F) in a dose-dependent manner. These results show that Andro and PDS significantly inhibited PRRSV replication in PAMs.
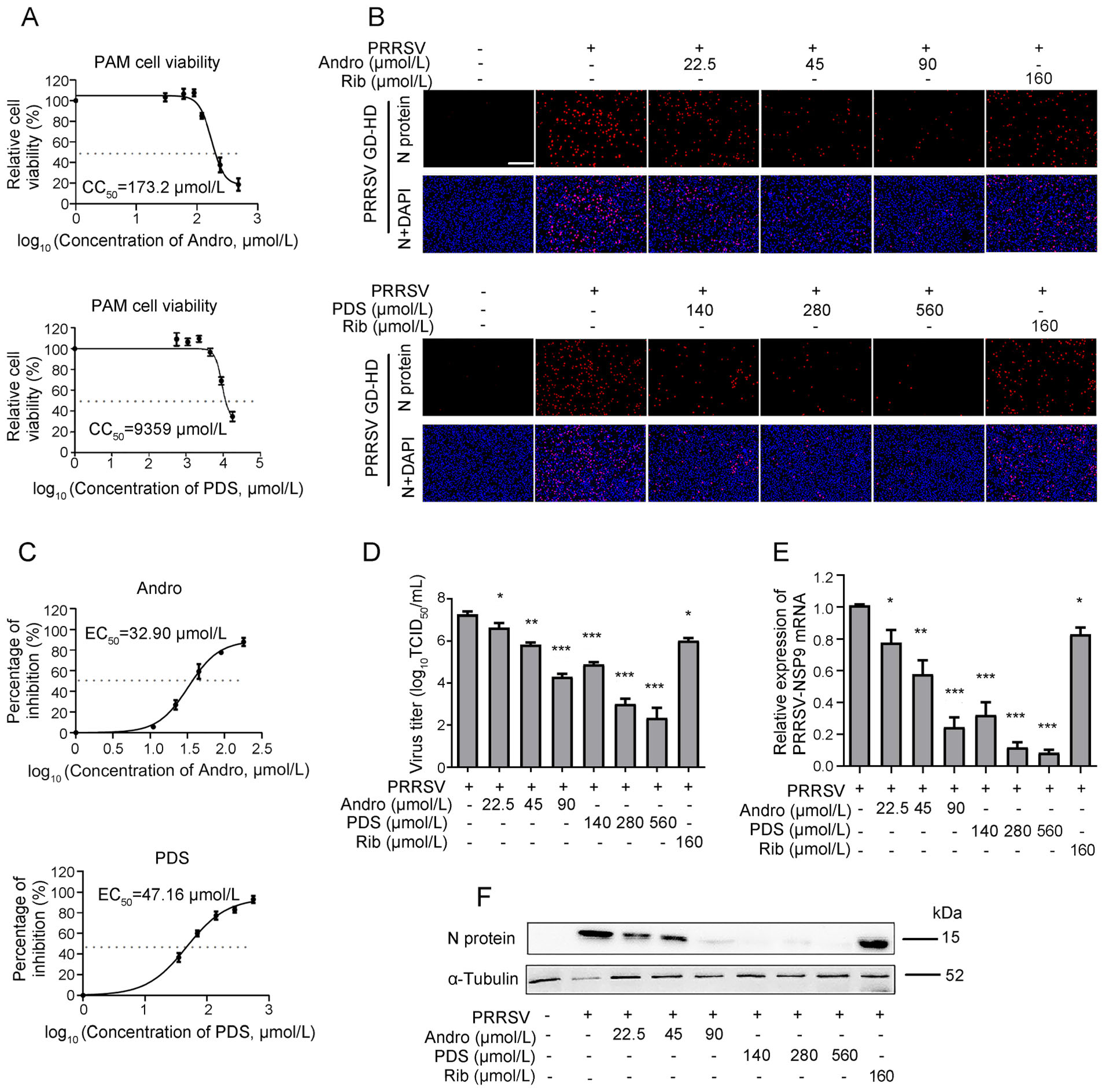
Figure 3. Cytotoxicity and anti-PRRSV activity of Andro and PDS in PAMs. (A) The cellular toxicity of Andro and PDS was examined in PAMs at 24 h using the MTT assay. PAMs grown in 24-well plates were infected with GD-HD PRRSV (1 MOI) for 2 h at 37 ℃ and then treated with various concentrations of Andro or PDS for 24 h. The expression of viral N protein was analyzed by IFA (B), and the percentage of inhibition based on the fluorescence optical densities (OD) of the images from three independent experiments are shown (C). Parallel samples were submitted for analysis of the viral titer using the end-point dilution assay (D), viral NSP9 gene mRNA level using qRT-PCR (E), and N protein level using Western blot (F). Scale bar: 250 μm. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the DMSO-treated control.
-
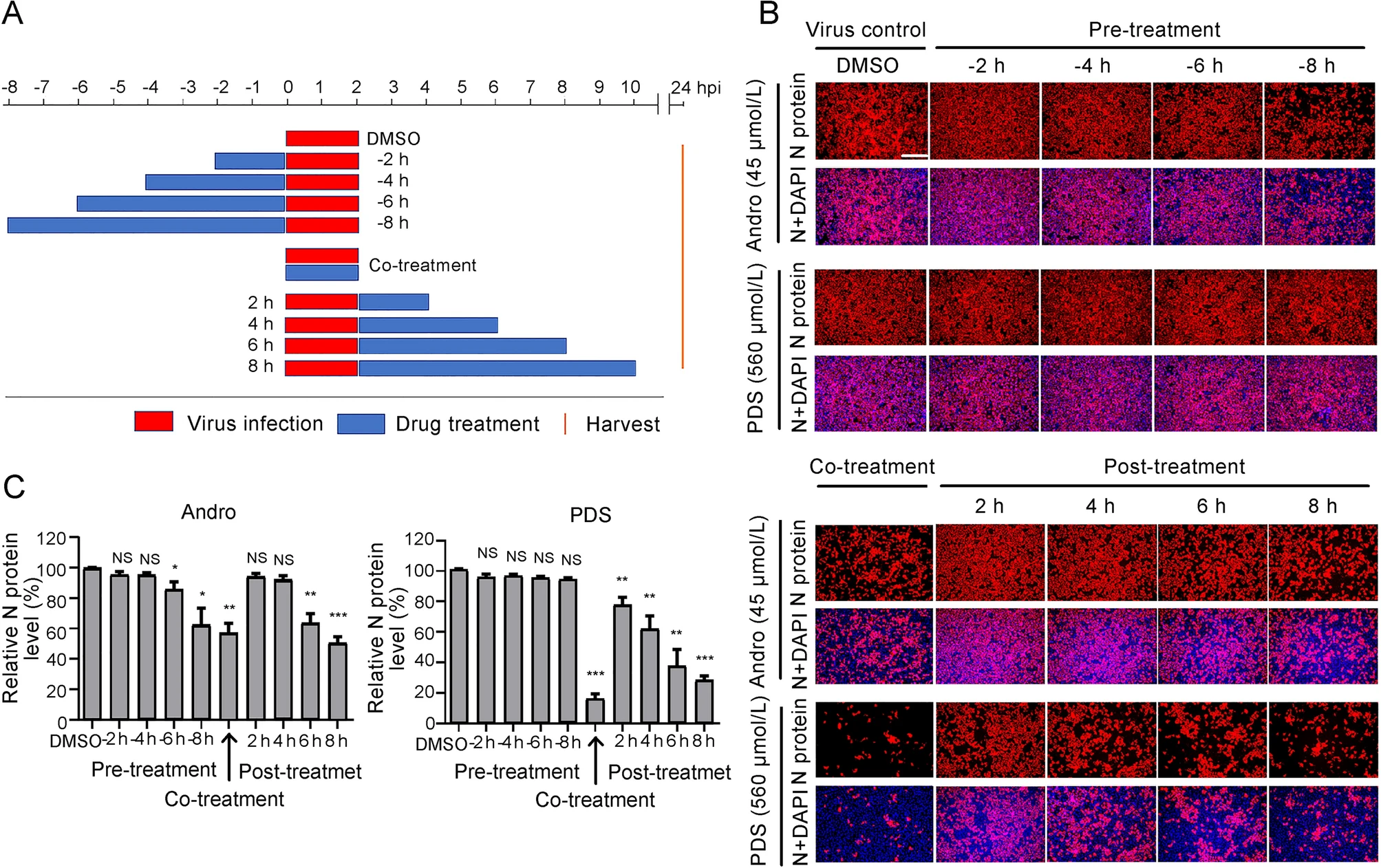
To identify the stage of the viral life cycle at which PRRSV replication is affected by Andro or PDS treatment, Marc-145 cells were treated with Andro at 45 μmol/L or PDS at 560 μmol/L before (pre-treatment), during (co-treatment), and after (post-treatment) PRRSV infection for different time period (Fig. 4A). At 24 hpi, cells were stained for IFA, the results show that pre-treatment with Andro for 2 h and 4 h did not reduce viral N protein production, while pre-treatment for 6–8 h partially reduced viral N protein production in a time-dependent manner (Fig. 4B). PDS did not reduce viral N protein production in the pre-treatment mode, indicating that PDS did not impair the susceptibility of Marc-145 cells to PRRSV infection. Notably, when the cells were treated with Andro or PDS during PRRSV infection (co-treatment) and post PRRSV infection, the viral N protein expressions were significantly suppressed in a time-dependent manner. In the co-treatment mode, PDS treatment resulted in an 82% decrease in N protein production compared to the virus control, while Andro treatment only reduced the viral N protein production by 43% (Fig. 4C). In the post-treatment mode, treatment with Andro for 6 h or 8 h led to a moderate decrease in viral N protein expression (38% and 46%, respectively), whereas treatment with PDS for 2–8 h led to a sharp decrease in N protein expressions in a time-dependent manner. PDS treatment for 6 h or 8 h led to a 63% or 70% decrease in viral N protein expressions, respectively, in the posttreatment mode (Fig. 4C). These results show that PDS exhibited stronger inhibition on PRRSV replication than Andro did in co- and post-treatments, but this profile was reversed in the pre-treatment mode, indicating that the two compounds likely inhibit PRRSV replication by differential mechanisms of action.
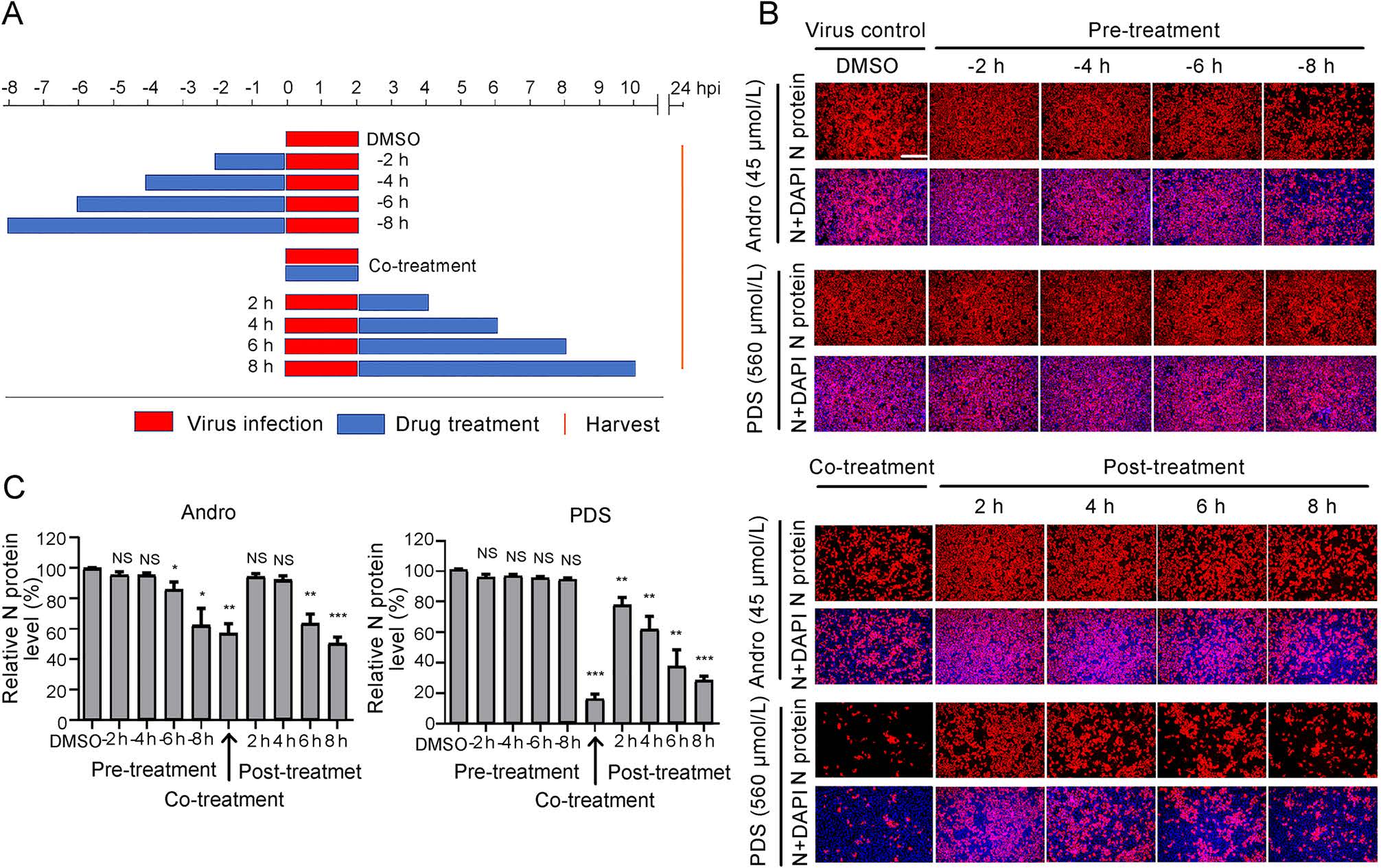
Figure 4. Andro and PDS suppress PRRSV replication in co- and posttreatment modes. Marc-145 cells were grown in 24-well plates for 24 h and then treated with Andro (45 μmol/L) or PDS (560 μmol/L) for 2–8 h prior to virus infection (pre-treatment), for 2 h during viral infection (co-treatment), or for 2–8 h after virus infection and removal (post-treatment) (A). For all three treatment modes, 0.05 MOI of PRRSV GD-HD was used to infect the cells for 2 h. At 24 hpi, the cells were subjected to viral N protein analysis using IFA (B). The percentages of inhibition based on the fluorescence optical densities (OD) of the images from three independent experiments are shown in C. Scale bar: 250 μm. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the DMSO-treated control.
-
As shown above, Andro and PDS exhibited potent inhibition on PRRSV replication in the co-treatment mode, which inspired us to investigate whether Andro and PDS were able to directly interact with PRRSV. To answer this question, PRRSV was incubated with various concentrations of Andro or PDS for 1 h at 37 ℃, followed by ultrafiltration to separate PRRSV from Andro or PDS. The PRRSV in the ultrafiltrate was resuspended to infect Marc-145 cells for 2 h, and then the cells were incubated in a fresh medium (Fig. 5A). At 48 hpi, the samples were collected to determine the viral N protein expression, virus titration, and viral gene mRNA levels. As shown in Fig. 5B and 5C, treatment with PDS led to a significant decrease in viral N protein expression in a dose-dependent manner. Treatment with PDS at 560 μmol/L resulted in an 80% reduction in viral N protein expression compared to that in the DMSO-treated control (Fig. 5C). However, Andro treatment did not lead to an obvious decrease in viral N protein expression, even at the highest concentration of 45 μmol/L. PDS and Andro exhibited similar PRRSV inhibition profiles in progeny virus production (Fig. 5D) and viral mRNA level (Fig. 5E) to those in N protein expression. These results suggest that PDS, but not Andro, directly interacted with PRRSV virion.
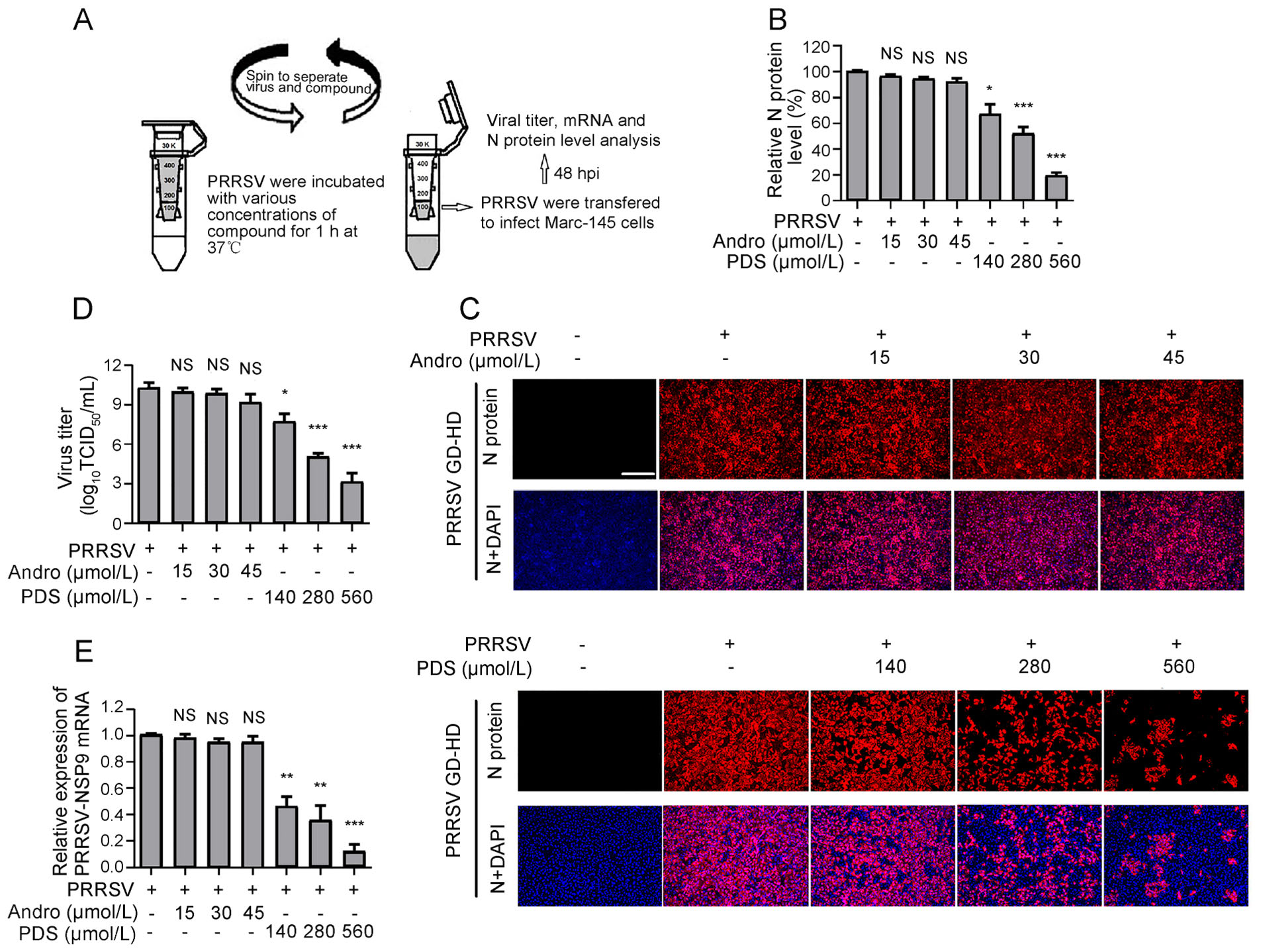
Figure 5. PDS rather than Andro interacts with PRRSV directly. 100 μL PRRSV GD-HD (2 × 106 TCID50) was mixed with various concentrations of Andro or PDS in essential medium (0.9 mL total volume) for 1 h at 37 ℃. Then, the PRRSV and compounds were separated by ultrafiltration, as shown in (A). Recovered PRRSV was resuspended and used to infect Marc-145 cells for 2 h, followed by incubation in fresh medium. At 48 hpi, samples were subjected to IFA for the detection of viral N protein (B and C). The images in C are representative results from the three independent IFA assays shown in B. Parallel samples were subjected for viral titration using the endpoint dilution assay (D) and viral mRNA analysis using qRT-PCR (E). Scale bar: 250 μm. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the DMSO-treated control. NS: No statistically significant difference compared to the control.
To investigate the impact of PDS treatment on PRRSV particles, PRRSV was incubated with PDS at different concentrations (280 μmol/L and 560 μmol/L) for 1 h at 37 ℃, subsequently, and the morphology of the virus particle was examined via transmission electron microscopy (TEM). Figure 6 shows that PDS incubation (280 μmol/L or 560 μmol/L) led to aggregation of virus particles, while PRRSV presented as discrete particles in the sample without PDS treatment. Meanwhile, aggregated virus particles lost clear morphological characteristics that were observed in the control sample. It can be speculated that the PDS treatment led to PRRSV inactivation and aggregation. These data further confirm the direct interaction between PDS and PRRSV.
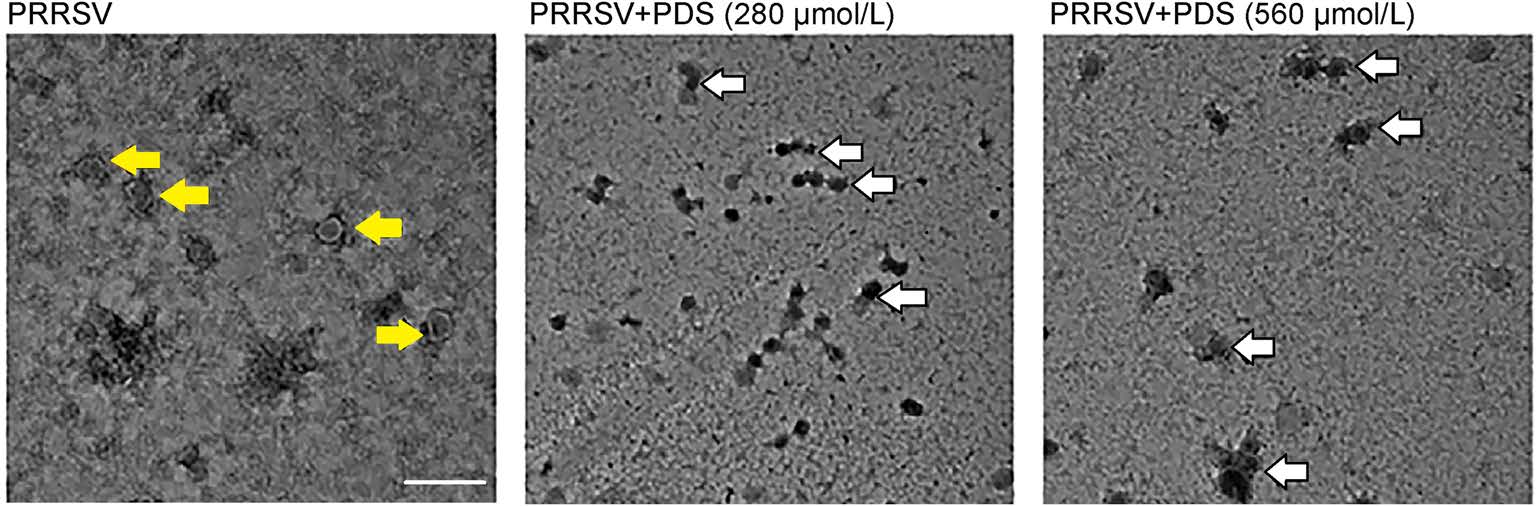
Figure 6. Electron micrographs of PDS-induced PRRSV aggregation. PRRSV (1 × 106 TCID50/mL) was treated with different concentrations of PDS (0, 280, and 560 μmol/L) for 1 h at 37 ℃; 2% PTA was used to stain the viral particles. The morphology of PRRSV virions was observed by transmission electron microscopy (TEM). The yellow arrows represent the virus particles. The white arrows represent the aggregation of virus particles. Scale bars: 200 nm.
-
PPRSV infection activates the NF-κB signaling pathway, which is an essential step for effective viral gene expression and replication (Hiscott et al. 2001; Wang et al. 2013). We further investigated whether Andro and PDS would inhibit PRRSV infection via affecting the activation of NF-κB in Marc-145 cells. Lipopolysaccharide (LPS), a wellknown NF-κB activator, was used in this experiment. Marc-145 cells were infected with 0.05 MOI of PRRSV for 2 h or stimulated with LPS (5 lg/mL) for 6 h at 37 ℃, and then treated with Andro or PDS for 24 h. Western blot and confocal microscopy were performed to evaluate the impact of Andro and PDS treatments on the activation of the NF-κB pathway induced by PRRSV infection or LPS stimulation. The results show that PRRSV infection and LPS stimulation effectively induced production of p-p65 and p-IκBα, which represent the activation of the NF-κB pathway, while treatment with Andro or PDS strongly inhibited IκBα degradation and reduced the production of p-IκBα and p-p65 (Fig. 7A). Consistently, the results of confocal microscopy analysis showed that Andro and PDS treatment effectively suppressed the nuclear translocation of p65 (Fig. 7B). Simultaneously, treatment with Andro or PDS potently suppressed PRRSV replication, reflected by decreased expression of viral N protein (Fig. 7A). Our results show that BAY11-7082, a specific NF-κB inhibitor exhibited a significant inhibition at 10 μmol/L on NF-κB activation induced by PRRSV infection in the same assays (Fig. 7A).
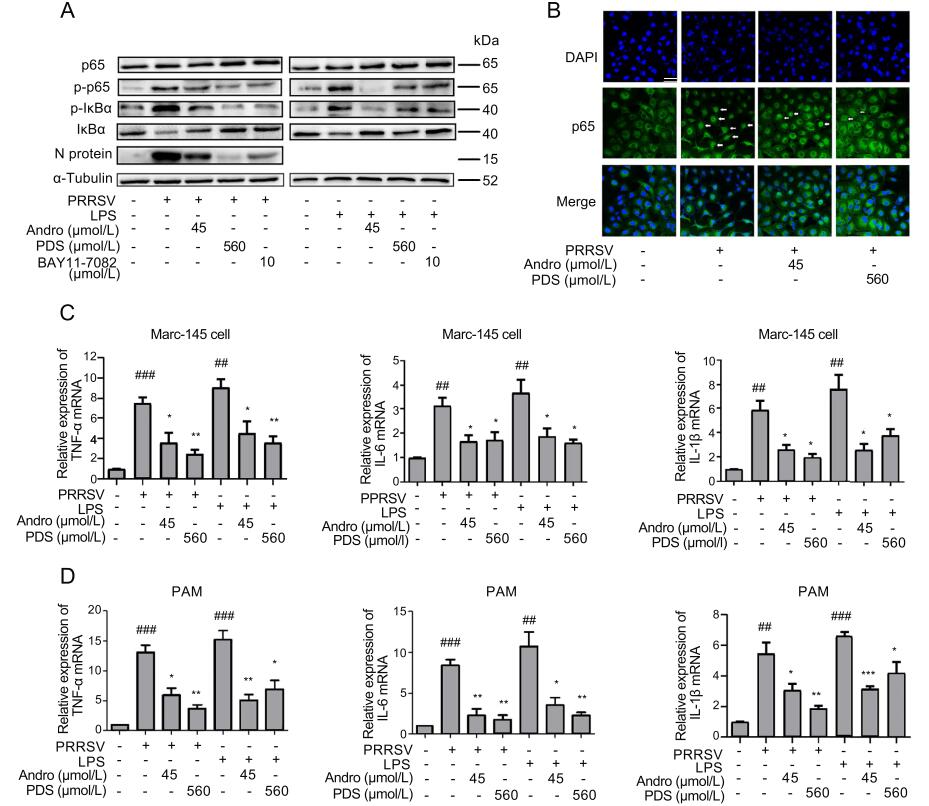
Figure 7. Andro and PDS inhibit NF-κB activation and subsequent production of pro-inflammatory cytokines in Marc-145 cells and PAMs. Marc-145 cells were infected with PRRSV GD-HD (0.05 MOI) for 2 h at 37 ℃ and treated with or without Andro (45 μmol/L) or PDS (560 μmol/L) for 24 h. Cells were inoculated with LPS (5 lg/ mL) at 37 ℃ for 6 h. (A) The protein expression of p65, p-p65, IκBα, and p-IκBα in Marc-145 cells treated with Andro or PDS was assessed by Western blot. (B) Confocal microscopy analysis of p65 nuclear translocation. At 24 hpi, cells were fixed and stained with Alexa Fluor 488-conjugated goat anti-rabbit anti-p65 IgG antibody (green). Nuclei were counterstained with DAPI (blue). The white arrows represent p65 nuclear translocation. Scale bar: 50 μm. (C and D) Effects of Andro and PDS on the production of TNF-α, IL-6, and IL-1β in PRRSV-infected and LPS-induced Marc-145 cells (C) and PAMs (D). Cells were collected to extract total RNA at 24 hpi. The relative expression of TNF-α, IL-6, and IL-1β was analyzed by qRTPCR. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the DMSO-treated control; #P < 0.05, ##P < 0.01, and ###P < 0.001 compared to the mock-infected control.
The activation of NF-κB can lead to the production of pro-inflammatory cytokines, which would deteriorate the pathological outcome of virus infections (Ke et al. 2019; Wang et al. 2019). To investigate whether Andro or PDS treatment affects cytokine expression, the mRNA levels of three pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, were analyzed in the presence and absence of Andro and PDS using qRT-PCR. The results shown in Fig. 7C demonstrate that Andro and PDS treatment significantly attenuated the mRNA expression of the three pro-inflammatory cytokines induced by PRRSV infection or LPS stimulation in both Marc-145 cells and PAMs.
-
In Marc-145 cells, PRRSV infection can induce oxidative stress in cells by generating ROS, which plays a key role in the pathogenesis of PRRSV (Guo et al. 2018). We further explored whether the inhibitory activity of Andro and PDS against PRRSV infection is related to its antioxidant activity. To this end, Marc-145 cells were infected with PRRSV for 2 h at 37 ℃ or stimulated with H2O2 at 37 ℃ for 0.5 h, and then incubated in a fresh medium in the presence or absence of Andro or PDS for 24 h. Samples were collected for ROS analysis by IFA and flow cytometry. The results in Fig. 8A and 8B show that PRRSV infection resulted in a significant increase in ROS and Andro or PDS treatment potently reduced ROS in infected cells. These antioxidative effects of Andro and PDS were also observed in H2O2-stimulated cells. MDA, a product of lipid peroxidation and a biomarker for estimating oxidative stress, was also dramatically reduced while treatment with Andro or PDS (Fig. 8C). Furthermore, the reduction in SOD and GSH induced by PRRSV infection were alleviated while treatment with Andro or PDS (Fig. 8D and 8E). These data indicate that Andro and PDS restored the oxidation balance in PRRSV-infected Marc-145 cells.
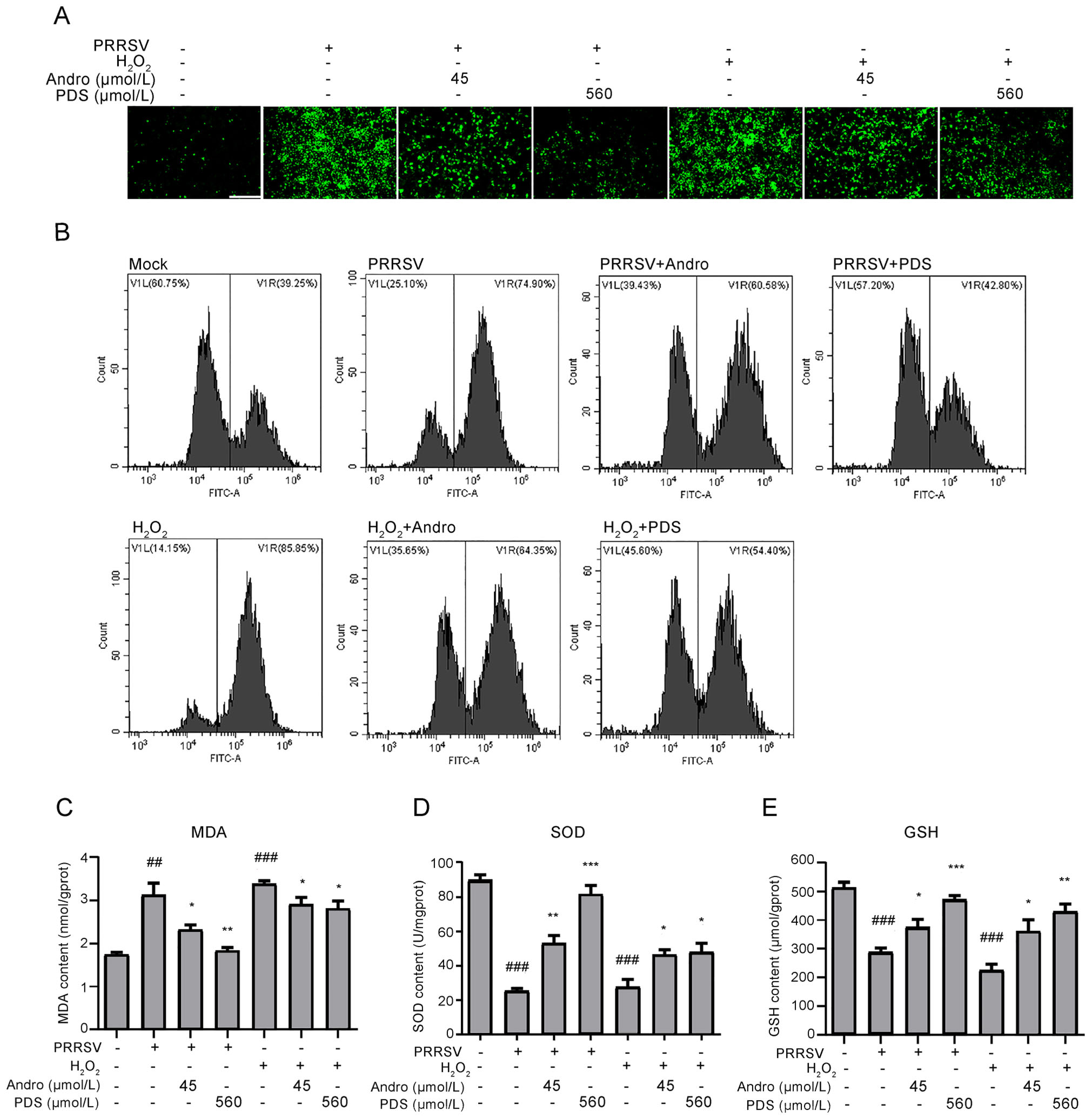
Figure 8. Andro and PDS suppress oxidative stress induced by PRRSV in Marc-145 cells. Marc-145 cells were infected with PRRSV (0.05 MOI) for 2 h at 37 ℃ and then incubated in fresh medium in the presence or absence of Andro (45 μmol/L) or PDS (560 μmol/L) for 24 h. As a positive control, Marc-145 cells were stimulated with H2O2 (2.5 μmol/L) at 37 ℃ for 0.5 h. (A and B) The ROS level, reflected by the ratio of positive cells stained with dichlorofluorescein (DCF), was determined using IFA (A) and flow cytometry (B). (C–E) Effects of Andro and PDS on the levels of MDA, SOD, and GSH in Marc-145 cells infected with PRRSV or stimulated with H2O2 were determined using their respective ELISA assay kits. Scale bar: 250 μm. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the DMSO-treated control; #P < 0.05, ##P < 0.01, and ###P < 0.001 compared to the mock control.
Andro and PDS Inhibit PRRSV Replication in Marc-145 Cells
Andro and PDS Inhibit PRRSV Replication in PAMs
Andro and PDS Inhibit PRRSV Replication in Coand Post-Treatment Modes
PDS Rather than Andro Directly Interacts with PRRSV Virions
Andro and PDS Suppress NF-κB Activation Induced by PRRSV Infection and Attenuate the Production of Pro-Inflammatory Cytokines
Andro and PDS Alleviate PRRSV-Induced Oxidative Stress
-
Although huge efforts have been made to control PRRS, PRRSV remains an economically important pathogen in the global pig industry. Current vaccines only provide limited protection to pigs from PRRS; therefore, new antiviral strategies against PRRSV infection are urgently required. Andro, a natural diterpene lactone, and its derivatives have been used clinically in China and among Asian countries for decades to treat inflammatory diseases, including bacterial and viral infections (Puri et al. 1993). In this study, we reveal that Andro and PDS possess potent antiviral activity against PRRSV infection and replication in vitro. Andro and PDS treatment resulted in a remarkable decrease in progeny viruses, both in Marc-145 cells and PAMs, at concentrations far below the detectable cell cytotoxicity threshold. Furthermore, we demonstrate that the anti-PRRSV activities of Andro and PDS are closely related to their inhibitory activities on NF-κB activation and oxidative stress induced by PRRSV infection. In addition, direct inactivation of PDS, but not Andro, on PRRSV was observed, suggesting that Andro and PDS perform through differential anti-PRRSV mechanisms. A schematic overview of the inhibitory cascade effect of Andro and PDS on PRRSV replication is shown in Fig. 9.

Figure 9. Schematic overview of the inhibitory cascade effect of Andro and PDS on PRRSV replication.
NF-κB is an inducible transcription factor that plays a key role in inflammatory, immune responses and regulation of cell proliferation or survival (Lawrence 2009). Under physiological conditions, NF-κB is sequestered in the cytoplasm as an inactive complex by its binding to a member of the inhibitory kappa B (IjB) family. Upon stimulation by a wide range of pro-inflammatory stimuli, the IjB proteins are phosphorylated by IjB kinase (IKK), which leads to disassembly of NF-κB-IjB complex (Yang et al. 1999). The subunit of NF-κB p65 is then phosphorylated, leading to nuclear translocation and subsequent binding of NF-κB to DNA regulatory elements of the target genes involved in various biological functions, such as expression of pro-inflammatory cytokines, e.g. IL-6, TNFa, and IL-1β (Baeuerle and Baltimore 1996). Although NF-κB has long been considered as a key transcription factor for the expression of a variety of antiviral cytokines (Hayden and Ghosh 2008), some viruses, including HIV-1, hepatitis B virus (HBV), cytomegalovirus (CMV), and influenza A virus (IAV), can directly activate and appropriate host cell NF-κB signaling to enhance viral replication (Hiscott et al. 2001; Williams et al. 2007; Asamitsu et al. 2008; Ludwig and Planz 2008). However, research results regarding the interplay between PRRSV and the NF-κB pathway have also been somewhat controversial. Earlier studies showed that persistent activation of NF-κB appears to be a general response to PRRSV infection (Lee and Kleiboeker 2005). Later studies showed that there are dual roles of NF-κB regulation by PRRSV: inhibition in early infection and activation later in the infection (Sun et al. 2012). It was also demonstrated that the optimal replication of PRRSV depends on the activation of NF-κB, and suppression of the NF-κB pathway could block PRRSV replication (Wang et al. 2013; Zhang et al. 2017). In this study, we found that Andro and PDS suppressed NF-κB activation in PRRSV-infected Marc-145 cells through both inhibition of NF-κB degradation and p65 nuclear translocation. Meanwhile, we also found that Andro and PDS reduced PRRSV N protein expression levels in MARC-145 cells, indicating the suppression of the two compounds on PRRSV replication. In addition, our data also demonstrate that Andro and PDS decreased NF-κB activation in LPS-treated cells (Fig. 7A and 7B), suggesting that the reduction of NF-κB activation in PRRSV-infected cells by Andro or PDS was not due to the reduction of virus infection. The specific NF-κB inhibitor BAY11- 7082 markedly suppressed NF-κB activation induced by PRRSV infection and decreased intracellular viral N protein expression levels, confirming that suppression of the NF-κB pathway could block PRRSV replication. Previous studies demonstrated that Andro exhibits anti-inflammatory activities via inhibiting NF-κB activation in vitro and in vivo (Zhu et al. 2013). Moreover, Xia et al. revealed that Andro attenuates inflammation by inhibiting NF-κB activation via covalent modification of reduced cysteine 62 in p50 (Xia et al. 2004). Therefore, although further studies are needed to explore the role of NF-κB in PRRSV infection, we can conclude that Andro and PDS indeed suppress PRRSV replication, either wholly or in part, by blocking NF-κB pathway activation.
PRRSV infection of pigs induces inflammation in the lungs, which is characterized by the increase of proinflammatory cytokines including IL-1β, IL-6, and TNF-α and by the recruitment of monocytes and neutrophils to infected sites (Van Reeth and Nauwynck 2000; Liu et al. 2010). It seems evident that PRRSV activates NF-κB and causes the NF-κB-mediated production of proinflammatory cytokines (Fu et al. 2012; Yu et al. 2016). In the early infection, activation of NF-κB leads to an intensive proinflammatory and type I interferon response, which is an effective mean of inhibiting PRRSV infection. However, persistent and excessive activation of NF-κB may lead overexpression and highly elevated levels of proinflammatory cytokines, which is closely correlated with PRRSV pathogenesis (Sun et al. 2012). Patricia et al. showed that PRRSV infection in pigs with a high-pathogenic strain induced a stronger systemic inflammatory response and more severe lung pathogenesis than that with a low pathogenic strain (Renson et al. 2017). In our study, we detected significantly elevated levels of three proinflammatory cytokines IL-6, TNF-α, and IL-1β at 24 hpi both in Marc-145 cells and PAMs, and treatment with Andro or PDS remarkably attenuated the production of three proinflammatory cytokines (Fig. 7C). Notably, the decrease in cytokine production might be a direct result of the anti-inflammatory activities of Andro or PDS, or an indirect result of their antiviral activities, or both. To identify these assumptions, we investigated whether Andro or PDS was able to inhibit NF-κB activation and cytokine expression in Marc-145 cells and PAMs stimulated by LPS in a parallel experiment. The results show that both Andro and PDS were capable of inhibiting LPS-induced NF-κB activation and cytokine expression as that induced by PRRSV infection. These results indicate that the inhibitory activities of Andro and PDS on NF-κB activation and cytokine expression are not PRRSV-specific. The anti-inflammatory effects of Andro against LPS stimulation in this study are consistent with the results of previous studies (Zhang et al. 2015; Li et al. 2017).
ROS plays a key role in many physiological and pathophysiological processes. The intracellular concentration of ROS is regulated by both free radical production and antioxidant defenses (Ray et al. 2012). At physiologic concentrations, endogenous ROS are essential signaling intermediates that regulate cell survival, growth, metabolism, and motility (Kumar et al. 2008). Increase in intracellular ROS following stimulation by a diverse range of stimuli can cause chronic oxidative stress and adverse effects, thereby injuring cells and inducing cell apoptosis and necrosis through damaging macromolecules, membranes, and DNA (Ray et al. 2012). It has been demonstrated that the generation of ROS is often involved in a wider range of virus infections, such as porcine circovirus type 2 (PCV2) (Chen et al. 2012), hepatitis C virus (Li et al. 2009), and herpes simplex virus (Gonzalez-Dosal et al. 2011). Previous studies have reported that PRRSV could down-regulate the expression of SOD, reduce that of GSH, and increase the production of ROS and MAD in Marc-145 cells, PAMs and the lung tissues of infected pigs (Liu et al. 2019). In addition, PRRSV can activate NF-κB signaling not only directly through viral proteins but also indirectly through increasing the level of ROS, which is beneficial for PRRSV replication. Indeed, in this study, PRRSV infection remarkably induced the generation of ROS, down-regulated the expression of SOD and GSH, and increased the concentration of MAD in Marc-145 cells. Furthermore, Andro and PDS treatment significantly reduced the production of ROS and MAD induced by PRRSV infection, and antagonized the PRRSV-induced decrease of SOD and GSH (Fig. 8). In addition, our data show that Andro and PDS treatment inhibited H2O2-induced oxidative stress in Marc-145 cells (Fig. 8), which is consistent with the results of previous studies in different cells (Xu et al. 2019). Taken together, our results suggest that Andro and PDS can inhibit ROS generation induced by PRRSV-infection and H2O2 stimulation.
Despite Andro and PDS inhibited PRRSV infection through suppressing virus-induced NF-κB activation and oxidative stress, whether there is direct interaction between the two compounds with PRRSV remains unclear. To resolve this, we designed an experiment to examine the inhibitory effect after co-incubation of Andro or PDS with PRRSV. Surprisingly, PDS, but not Andro, showed robust direct inactivation of PRRSV (Fig. 5). This effect of PDS was confirmed by the results from TEM analysis, in which normal PRRSV particles presented in a dispersed state and a large number of PDS-treated PRRSV particles were aggregated (Fig. 6), which is known to represent disability of infection (Welsh et al. 1976). However, the binding site of PDS on PRRSV remains unclear. As PDS treatment resulted in the aggregation of PRRSV particles, PDS likely binds to one of the PRRSV surface molecules, such as E and GP2 to GP5, directly affecting PRRSV binding to its entry receptors, including heparan sulphate, sialoadhesin, CD163, and others (Van et al. 2010).
Since the lactone moiety and its conjugated double bond is the core structure of Andro and its analogues, which is the source of their pharmacological activities (Nanduri et al. 2004), PDS differs from Andro in the hydroxyl group at C-14 in the lactone moiety and esterification of hydroxyl groups at C-3 and C-19 with succinic acid. These structural differences may attribute to differential antiviral mechanisms and different cytotoxic activities between Andro and PDS. In this study, PDS exhibited lower anti-PRRSV activity and much lower cytotoxicity than Andro both in Marc-145 and PAM cells. Furthermore, Chen et al. synthesized a series of dehydroandrographolide derivatives and showed that the free hydroxyl group at C-3 was necessary for maintaining high anti-HBV activity (Chen et al. 2014). In addition, Yu et al. showed that the cytotoxicity of dehydroandrographolide derivatives in human renal tubular epithelial cell (HK-2) was significantly lower than that of Andro (Yu et al. 2019). Based on previous studies on the structure–activity relationship of Andro derivatives, it could be speculated that dehydration of the hydroxyl group at C-14 in Andro results in the lower cytotoxicity of PDS, while esterification of the hydroxyl groups at C-3 in Andro leads to the lower antiviral activity of PDS. However, the accurate explanation for the difference in anti-PRRSV activity and cytotoxicity between Andro and PDS should be elicited based on further studies on their structure–activity relationship.
In summary, our study shows that Andro and PDS, two of the most commonly used anti-inflammatory drugs in China and among Asian countries, robustly suppress PRRSV replication in cell cultures by differential antiviral mechanisms. Considering that Andro and PDS are commercially available drugs with well-tolerated characteristics, they have the potential to pave novel therapeutic opportunities for combating PRRSV infection. in vivo evaluation is necessary to approve Andro and PDS as effective PPRSV inhibitors in swine.
-
This work was funded by the National Key Research and Development Program of China (Grant 2017YFD0501404), the National Natural Science Foundation of China (Grant 31872521), and the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (Grant 2019BT02N054) and the Basic Research & Applying Basic Research Foundation of Guangdong Province (Grant 2019B1515210007).
-
JC, TA, and LC conceived and designed the study. Experiments were performed by LS, YG, MZ, ZL, and QL. Data were analyzed by LS, LG, and JG. LS and JC wrote the manuscript. All authors read and approved the manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.

















 DownLoad:
DownLoad: