-
Chronic hepatitis C virus (HCV) infection is a progressive disease that may end in liver cirrhosis and hepatocellular carcinoma (2). Interferon-α (IFN-α) alone or in combination with ribavirin is the only currently approved treatment for HCV infection (5). The activity against viruses of IFN is mainly mediated via the Jak-STAT (signal transducer and activator of transcription) signaling pathway. Following binding of IFN to the IFN receptor complex, Jak1 and Tyk2 kinases are activated and in turn phosphorylate STAT1 and STAT2. Phosphorylated STAT1 (P-STAT1), toge-ther with phosphorylated STAT2, and interferon regu-latory factor 9 (IRF-9) form a heterotrimeric complex, IFN-stimulated gene (ISG) factor 3 (ISGF3). ISGF3 then binds to the interferon-stimulated response element (ISRE) sequence in ISGs promoters. Thus, several antiviral proteins are induced, such as MxA, double-stranded RNA-activated protein kinase (PKR), and 2'-5'oligoadenylate synthetase (2'-5'OAS) (7, 13). Previous studies show that the activated Jak/STAT pathway was well balanced by another family of negative regulators, suppressors of cytokine signaling (SOCSs), including SOCS1 and SOCS3, which affect cytokine signaling by inhibiting phosphorylation of STAT factors (12).
HCV core protein, which is involved in forming the viral nucleocapsids, can affect a whole array of host cell function, including apoptosis, cell transformation, signal transduction, and transcriptional regulation (8, 10, 11). However, the role of HCV core protein in modulating IFN-induced ISGs expression is more controversial. Data from different groups indicate that core protein modulates the IFN-induced Jak/STAT signaling pathway but does not affect the activation of some ISGs (1). In contrast, other authors have re-ported HCV core protein mediated activation of 2'-5'OAS gene transcription (9). Thus, whether HCV core protein interferes with expression of IFN-induced antiviral genes and whether this interference is involved in the activation and negative regulation of Jak/STAT signaling pathway remain to be demon-strated.
HTML
-
The plasmid pCI-core, in which the complete coding region of HCV core protein (1a genotype) was cloned into vector pCI (Promega, USA), and pISRE-luc, containing the ISRE-directed luciferase reporter gene were all from our laboratory. The antibodies used in this study included human anti-HCV positive serum obtained from Tongji Hospital, mouse anti-SOCS3 IgG (Biolegend), rabbit anti-STAT1 IgG, and rabbit anti-phospho-STAT1 (Tyr 701) IgG (Santa Cruz).
-
The human hepatoblastoma cell line HepG2 was used in the present study. Cells were propagated in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Hyclone), streptomycin (0.1mg/mL) and penicillin (100U/mL), at 37℃ in a atmosphere with 5% CO2. All transfections of expression plasmids were performed in 12-well plates using lipofectamine 2000 (Invitrogen, USA) according to the manufacturer's instructions.
-
Total cellular RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's protocol. RNA was reverse-transcribed using oligo (dT18) primers. Equal amounts of cDNA were subjected to PCR. The primer pairs used were following.
for GAPDH:
5'-TGATGACATCAAGAAGGTGG-3' (sense)
5'-TTACTCCTTGGAGGCCATGT-3' (antisense)
for SOCS3:
5'-CTCAAGACCTTCAGCTCCAA-3' (sense)
5'-TTCTCATAGGAGTCCAGGTG-3' (antisense)
for PKR:
5'-CCAGTGATGATTCTCTTGAGAGC-3'(sense)
5'-CCCCAAAGCGTAGAGGTCCA-3' (antisense)
for MxA:
5'-ATCGACCTCATTGACTCCCT-3' (sense)
5'-TGATTGCCCACAGCCACTCT-3' (antisense) for 2'-5'OAS:
5'-GGTGGTAAAGGGTGGCTCCTC-3' (sense)
5'-TCTGCAGGTAGGTGCACTCC-3' (antisense)
-
Cells were washed with phosphate-buffered saline (PBS) and lysed in lysis buffer (30mmol/L Tris, pH7.4, 150mmol/L sodium chloride, 1mmol/L phenylmethyl-sulfonyl fluoride, 1mmol/L sodium orthovanadate, 1% Nonidet P-40, 10% glycerol) for 30min on ice, vortexed, and centrifuged at 12 000 r/min at 4℃ for 10min. The supernatants were mixed in SDS loading buffer, boiled for 5min, and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred onto nitrocellulose membranes and blotted with newborn bovine serum (10%) for 12h. Membranes were incubated with primary antibodies for 1h and washed with PBS containing 0.1% (v/v) Tween-20, then incubated with horseradish peroxidase-conjugated secondary antibodies for 45min. Protein bands were visualized by an enhanced chemiluninescene reaction (Promega).
-
To monitor IFN signaling directed by the ISRE, the plasmids pISRE-luc (0.5μg), expressing firefly luci-ferase were cotransfected with plasmid pCI-core (1μg) or pCI (1μg), then cells were stimulated with IFN-α for 24h. Relative luciferase activity was assessed by the Promega luciferase reporter assay system.
Plasmids and reagents
Cell culture and transfection
RNA purification and RT-PCR
SDS-PAGE and Western blot analysis
Luciferase activity assay
-
Plasmid pCI-core and pCI was transiently trans-fected into HepG2 cells. Expression of HCV core protein was detected by Western-blotting (Fig.1A). To examine whether HCV core protein affected the expression of IFN-induced ISGs, we transfected pCI-core or pCI and treated the cells for 24h with 1 000IU/mL IFN-α. After that, total mRNA was isolated, and mRNA levels of PKR, MxA and 2'-5'OAS were semiquantitated by RT-PCR. As shown in Fig. 1B to D, treatment of IFN-α resulted in induction of transcription of PKR, MxA and 2'-5'OAS in pCI-transfected HepG2 cells. However, the mRNA levels of these genes reduced in a dose-dependent manner in pCI-core-tansfected cells compared to pCI-transfected cells.
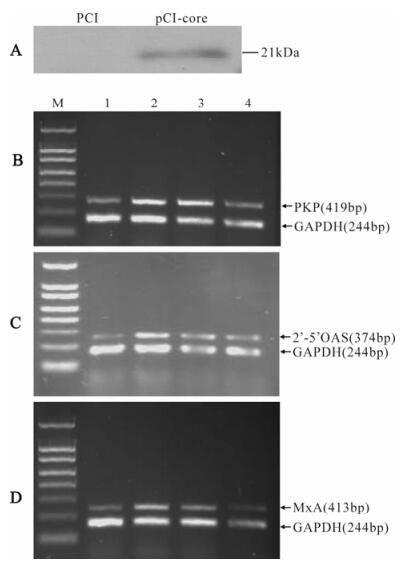
Figure 1. Inhibition of PKR, MxA and 2'-5'OAS mRNA level by HCV core protein. A: Western-blotting analysis of HCV core protein expressed in HepG2 cells. B, C and D: Levels of PKR, MxA, 2'-5'OAS and GAPDH mRNA were determined in HepG2 cells transfected with pCI or pCI-core and treated for 24h with 1 000U/mL IFN-α. M, 150bp DNA ladder. Lane 1, untreated HepG2 cells transfected with 2μg of pCI. Lane 2, IFN-treated HepG2 cells transfected with 2μg of pCI. Lane 3, IFN-treated HepG2 cells transfected with 1μg of pCI-core and 1μg of pCI. Lane 4, IFN-treated HepG2 cells transfected with 2μg of pCI-core.
-
To test whether the observed effect of HCV core on the reduction of ISGs expression is mediated by ISRE, HepG2 cells were cotransfected with pCI-core and pISRE-luc, and treated with different concentrations of IFN-α. Luciferase activity detection showed that IFN-induced ISRE-mediated gene expression was inhibited in the cells expressing HCV core protein (Fig.2).
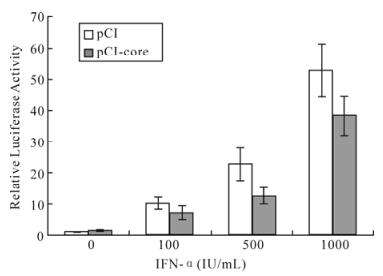
Figure 2. HCV core protein inhibits ISRE-mediated signaling in luciferase assay. Plasmid pCI-core or pCI with pISRE-luc luciferase reporter gene containing an ISRE element were cotransfected to HepG2 cells with IFN-α (0, 100, 500, 1 000 IU/mL) treatment for 24 h. Total protein was extracted for luciferase assay. pCI-core: pCI-core+pISRE-luc. pCI: pCI+ pISRE-luc.
-
To address whether HCV core protein inhibits ISRE-mediated ISGs expression via Jak/STAT signal transduction, after transfected with pCI-core or pCI, and treatment with or without IFN-α for 30min, HepG2 cells were assayed for the levels of STAT1 and P-STAT1 using western-blotting. As shown in Fig. 3, HCV core expression had no effect on STAT1 levels, but was associated with significantly decreased P-STAT1 level.
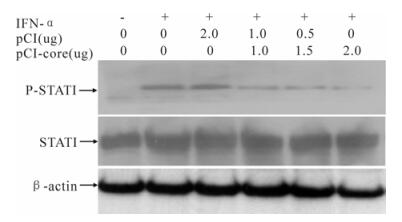
Figure 3. Inhibition of HCV core protein on phosphorylation of STAT1. HepG2 cells were transfected with different amounts of plasmid pCI-core or pCI for 48h, and treated with or without 1 000 IU/mL IFN-α for 30min. Total protein extracted from the cells was analyzed by Western-blotting using anti-STAT1 or anti-P-STAT1. Actin protein was used as a control.
-
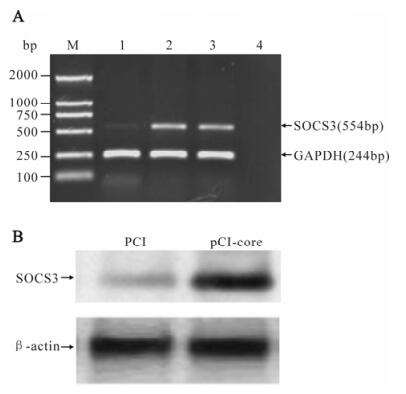
To understand whether SOCS3 involves the inter-ference of HCV core protein on Jak/STAT signal transduction, we examine the effect of HCV core protein on SOCS3 expression by RT-PCR and We-stern-blotting. The results showed that mRNA and protein levels of SOCS3 in core-transfected cells are induced than in cells transfected with empty vector group (Fig.4).
Effect of HCV core protein on the transcription of PKR, MxA and 2'-5'OAS in HepG2 cells
HCV core protein inhibits ISRE-mediated reporter gene expression
HCV core protein inhibits IFN-induced P-STAT1
HCV core protein activates the expression of SOCS3
-
IFN-α is one of the most potent innate antiviral cytokines. Its activity against viruses, such as HCV, is believed to depend both on the activation of many ISGs and on modulation of the immune system. Unfortunately, the best sustained virologic response rate to IFN-α in chronically infected individual is less than 50% (5). The failure of viral clearance and the frequent nonresponsiveness to IFN treatment suggest that HCV, like many other viruses, has evolved several mechanisms to disarm the innate immune response. HCV protein expression has been shown to antagonize IFN-stimulated genes in vitro. For example, the NS3/4A protease functions as an antagonist of virus-induced IRF-3 to impair IFN production in hepatocytes and thereby inhibits the expression of several ISGs (4). The structural protein E2 and the nonstructural protein NS5A modulate interferon responses by inhibiting the interferon inducible PKR (14, 6). HCV core protein was shown to repress IRF-1 expression, which results in inhibition of type I IFN production and down-regulation of the transcription of several ISGs, such as IL-15 and IL-12 (3). Basu et al. have reported that expression of HCV core protein induces a reduction in formation of the ISGF3 complex, whereas the activation of IFN stimulated genes IRF-1 and 561 were not affectted (1). In contrast, Naganuma et al. have shown that HCV core protein activates transcription of the inducible 2'-5'OAS gene in transgenic mice (9). Thus, whether HCV core protein interfere with the transcription of IFN-inducible genes remains controversial. In the present study, our results showed that, in the presence of the core protein, mRNA levels of PKR, MxA, and 2'-5'OAS, which are important IFN-inducible anti-viral molecules, decreased in a dose-dependent manner in HepG2 cells, suggesting that core protein might inhibit IFN-α inducible transcription of ISGs. However, these results are in contrast with those reported by Naganuma et al. This difference may be due to differences in the experimental systems used.
ISRE sequence exists in the promoters of many ISGs, including PKR, MxA, and 2'-5'OAS. Our results showed that the expression of luciferase reporter gene containing ISRE sequece was inhibited by HCV core protein after treatment with IFN-α, which indicated that the observed effect of HCV core on the reduction of ISGs levels might be mediated by ISRE. Previously studies showed that IFN-induced ISGs expression is mainly through the activation of the Jak/STAT signaling pathway, in which STAT1 phosphorylation is a critical step. In this study, we found that following treatment with IFN-α, HCV core protein had no effect on STAT1 expression but depressed STAT1 tyrosine phosphorylation levels, which suggested that the inhibition of Jak/STAT activation might be the cause of HCV core protein down-regulating IFN-α inducible ISGs expression.
SOCS3 is an important negative regulator of the Jak/STAT pathway by inhibiting phosphorylation of STAT1. Recent studies show that in liver tissues of chronic HCV infected individual, the SOCS3 level is much higher in non-responders to IFN-α than that in responders (16). Another group reported an enhanced SOCS3 response in the IFN-resistant HCV sub-genomic replicon cells compared with IFN-sensitive cells (15). These results suggest that SOCS3 may be involved in the process of HCV resisting IFN-α response. We examined whether HCV core protein inhibited IFN-α inducible ISGs expression via up-regulating SOCS3 level. RT-PCR and Western-blotting were used to analyze the effect of the core protein on the transcription and expression of SOCS3. The results showed that HCV core protein could induce the expression of SOCS3 in HepG2 cells, and enhanced SOCS3 expression might be responsible for the HCV core protein repressing IFN-α mediated ISGs expression.
In summary, our studies show that HCV core protein can down-regulate the expression of interferon-induced antiviral genes, which may involve the inhibition of STAT1 phosphorylation and the induc-tion of SOCS3. It suggests that HCV core protein may play an important role in blocking type I IFN signal transduction and contributes to viral persistence. Further studies to determine whether HCV core protein mediates the interference of HCV with IFN signaling in vivo will be of interest.














 DownLoad:
DownLoad: