-
Baculovirus has been widely used for production of recombinant proteins in insect cells. Previous studies found when a reporter gene under the control of a mammalian promoter was cloned into the baculovirus genome, it was expressed in hepatoma cells (Huh7 and HepG2), primary rat hepatocytes, epithelioid cell lines and rabbit intervertebral disc cells (Hela and Cos7) in vitro (2, 7, 14). Since these findings demonstrated that baculovirus can efficiently transduce mammalian cells, the applications of baculovirus have been greatly expanded (23). Interestingly, although baculovirus can transduce mammalian cells very efficiently it only replicates in insect cells (10). Therefore, it has been suggested that baculovirus could be used as a gene therapy vector (11, 12).
Baculovirus p35 gene is from the baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) and is known to inhibit cell apoptosis in different organisms as diverse as Caenorhabditts elegans, Drosophila melanogaster and mammals, including humans (1, 4). Expression of p35 prevents apoptosis induced by various stimuli in carcinoma cells, neurons, oligdendrocytes, vascular smooth muscle cell and pancreatic beta-cell (8, 17, 19). Compared with cardiomyocytos from wild-type mice, cardiomyocytes from p35 expressing transgenic mice had longer lifetime under hypoxia conditions, and markedly reduced cytochrome C release, and caspase 3 acti-vation. Additionally, purified P35 has been found to inhibit all the mammalian caspases except caspases 5 and 9 (20). Based on previous studies, the molecular mechanism of inhibiting apoptosis is most likely the direct inactivation of caspase by P35 protein. Because of these characteristics, P35 is also commonly referred to as a pan-caspase inhibitor (21). In this study, we investigated whether the expression of p35 gene mediated by baculovirus vector can protect human embryo kidney 293 cells against apoptosis. The results demonstrated that baculovirus-mediated expression of p35 effectively protected human embryo kidney 293 cells against apoptosis induced by various apoptosis inducers such as Actinomycin D, UV and serum-free media, and it could be widely used in molecular research and even gene therapy.
HTML
-
The Spodoptera frugiperda (Sf9) cells were main-tained at 28℃ in Grace's media supplemented with 10% fetal bovine serum (GiBco). Mammalian cell line human embryo kidney 293 was obtained from the China Center for Type Culture Collection, Wuhan (CCTCC) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin (GiBco), at 37℃.
-
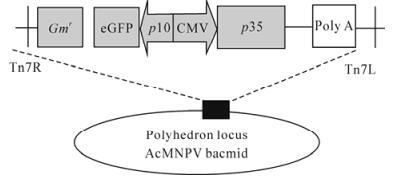
The baculovirus vectors were constructed as described previously. The p35 gene was cloned by PCR from baculovirus genomic DNA. A ~1.0 kb EcoR I p35 fragment was inserted into the EcoR Ι site of pFBDeGFPCMV (which contains the human cytomegalovirus enhancer/promoter, polyadenylation signal from SV40, eGFP gene was driven by P10 promoter) (15). The correct construct was confirmed by PCR. With the donor plasmid pFBDGCp35, recombinant virus Ac-CMV-p35 was generated using the Bac-to-Bac system according to the manufacturer's protocols (Life Technologies). Viral titers were deter-mined by the end-point dilution using Sf9 cells. Ac-CMV-GFP was used as a control (12).
-
Cells were seeded in 35 mm culture dishes at 5×105 cells per dish. Culture medium was removed, replaced with virus, and incubated for 2 h at 37℃. After removal of the virus, fresh medium was added and cultures were incubated at 37℃. Cultures were harvested, washed, and resuspended in phosphate-buffered saline. The GFP-expressing populations were analyzed by FACS (Beckman).
-
3-(4, 5-Dimethythiazal-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay was used to estimate cell survival by quantifying the total cell numbers. In this assay, 293 cells (2×104 cells/well) were seeded into a 96-well plate. Culture medium was removed, replaced with virus, and incubated for 2 h at 37℃. After removal of the virus, fresh medium was added and the cultures were incubated. After 72 h treatment, the medium was replaced by 100μL/well normal medium containing 0.5 mg/mL MTT (sigma). Cells were incubated at 37℃ for 4 h. Then the medium containing MTT was removed and 100 μL/well 100% DMSO was added. After formazan was dissolved completed, the plate was placed on the microplate reader (Bio-Rad) to measure optical density at 570 nm.
-
Cell death was studied morphologically using a DNA dye, Hoechst 33258, which stains nuclei. Apoptotic cells have condensed chromatin and nuclear fragmentation that produces a characteristic, irregular staining of their nuclei, its chromosomes fluoresces brightly. 293 cells treated with different apoptosis-inducing stimuli such as Actinomycin D or UV radiation (w312 μmol/L, 1.8×10-6 watts/cm2 at lamp to target distance). The percentage of apoptotic cell death was assessed by Hoechst 33258 staining according to the manufacturer's protocols (Bi Yuntian Company, Haimen, China). Starvation was induced by exposure to free serum cell culture and the survival rate was calculated by trypan blue, a vital dye, staining. Under a microscope, observe if non-viable are stained and viable cells excluded the stain.
-
Cells were fixed in 4% paraformaldehyde at 72 h post transduction and processed for immunofluore-scence. P35 was visualized by indirect immunofluore-scence using rabbit-anti-p35 primary antibody (BD Biosciences) diluted 1:200. The bound antibody was visualized with FITC-conjugated goat-anti-rabbit IgG (San Ying Company, Wuhan, China) diluted 1:200 secondary antibody. Slides can be stored in the fridge or freezer until analyzed by confocal microscopy (Leica, Heidelberg, Germany).
Cell culture
Construction of recombinant baculovirus vector
Transduction of mammalian cells by baculovirus
MTT assay
Analysis of apoptotic cell death
Immunofluorescence analysis
-
Recombinant viruses Ac-CMV-p35 were generated using the Bac-to-Bac system (Fig. 1). After tansfection and infection, viral titers were determined by the end-point dilution with Sf9 cells. The titer of recombinant viruses Ac-CMV-p35 is 108 PFU/mL.
-
Expression of eGFP in mammalian 293 cells was investigated by FACS analysis. These cells were transduced with Ac-CMV-GFP (12) (with a series of MOI) following the addition of a deacetylase inhibitor, butyrate. The cells were harvested 48 h later. The percentage of cells expressing GFP was detected at different MOIs. With the addition of 10 mmol/L sodium butyrate, the percentage of eGFP-expressing cells expressing GFP increased as the MOI increased. The highest percentage was 86.6 ± 2.26% when using 200 MOI baculovirus (Fig. 2). This result indicated that recombinant baculovirus vectors are capable of transducing 293 cells efficiently.
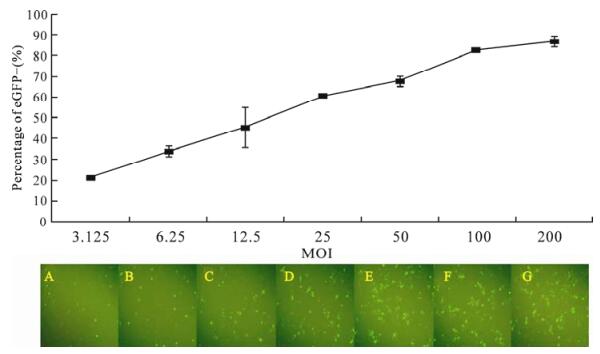
Figure 2. Baculovirus-mediated GFP gene expression. 293 cells were transducted with Ac-CMV-GFP at 3.125 (A), 6.25 (B), 12.5 (C), 25 (D), 50 (E), 100 (F), 200 (G) MOI for 2 h, and percentages of GFP+ cells were detected at 48 h post-transduction. Results are shown as the mean ±standard error of 3 independent experiments.
-
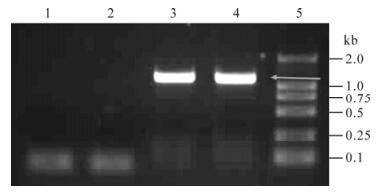
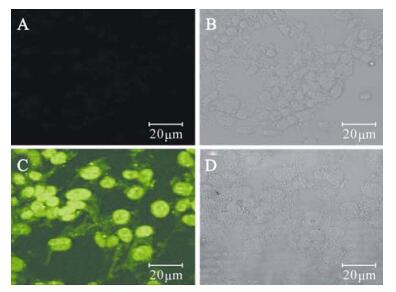
The recombinant baculovirus Ac-CMV-p35 (MOI= 100) was transduced into 293 cells. At 24 h, the transcription of p35 gene was detected by reverse transcriptase polymerase chain reaction (RT-PCR). The 1 kb fragment was only found in the sample of transduced 293 cells indicating the successful trans-cription of the p35 gene in 293 cells (Fig. 3). Further evidence of p35 expression in transduced cells was found at the protein level by immunostaining 293 cells with an anti-p35 antibody (Fig. 4). The transduced cells showed staining for p35 in the intact cells in both the cytoplasm and the nucleus.
-
Transduction of recombinant baculovirus vector is not toxic to mammalian cells. Transduction with Ac-CMV-p35 up to a MOI of 200 did not reduce cell viability, as measured by the MTT assy. MTT was also performed to estimate the effect of baculovirus-mediated expression of p35 on mammalian cell proliferation. The viability of 293 cells transduced by Ac-CMV-GFP and Ac-CMV-p35 was not changed more than 5% (Fig. 5). In addition, compared to time-matched controls, there was no difference in the growth curve of cells transduced by Ac-CMV-GFP or Ac-CMV-p35 for 7 d (Fig. 6). Cell viability and cell growth curve assays demonstrated baculovirus-me-diated expression of p35 had no toxic effects on mammalian cell viability and growth.
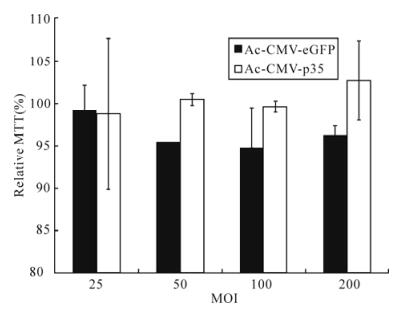
Figure 5. Cytotoxic effects analysis by an MTT assay. 293 cells were seeded in 96-wells culture dish at 2.0×104 cells per well and transducted with Ac-CMV-GFP and Ac-CMV-p35 at 100 MOI. Cell survival was quantified by the MTT assay after 72 h post-transduction. Results are expressed as the mean ± standard error of 3 independent experiments.
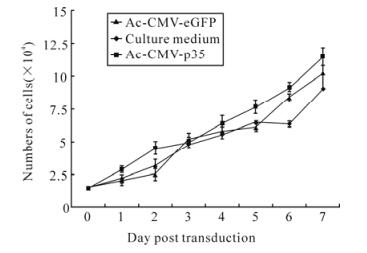
Figure 6. Growth curve of 293 cells transducted by baculovirus. 293 cells were seeded in 96-wells culture dish at 2.0×104 cells per well, and transducted with Ac-CMV-GFP and Ac-CMV-p35 at 100 MOI for 2 h, Numbers of trypan blue negative cells were counted manually using a haemocytometer. Results are expressed as the mean ± standard error of 3 independent experiments.
-
To assess the effect of baculovirus-mediated expres-sion of p35 on 293 cells resistance to apoptosis, the percent apoptosis cells induced by Actinomycin D was determined by Hoechst 33258 staining assay. The percentage of apoptotic cells decreased 34.14±7.05% and 64.51±4.86%, respectively, by using 12.5 MOI and 25 MOI Ac-CMV-p35 compared with the control baculovirus Ac-CMV-eGFP. The percentage of apop-totic cells with 50 MOI Ac-CMV-p35 decreased approximately 2-fold than that with 12.5 MOI Ac-CMV-p35 (Fig. 7). However, the efficiency of apop-tosis inhibition could not increase correspondingly by using the higher MOI Ac-CMV-p35 once MOI was more than 50 MOI.
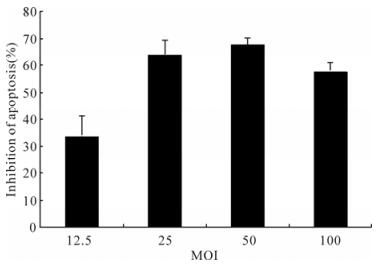
Figure 7. MOI of baculovirus-mediated p35 gene expression and resistance of 293 cells apoptosis induced by ActD. Cells were transducted with Ac-CMV-GFP and Ac-CMV-p35 at 12.5, 25, 50, 100 MOI for 2 h, and cells were induced by 0.25 mmol/L ActD after 36 h post-transduction. Percentages of apoptosis cells were detected by Hochest 33258 staining after 54 h. To calculate relative inhibition, apoptosis cells of duplicate samples were compared to Ac-CMV-GFP and were multiplied by 100.
-
To verify that baculvirus-mediated expression of p35 could inhibit apoptosis mediated by other inducers, the inhibition of apoptosis induced by UV was determined. Antiapoptosis assay showed that 25 MOI Ac-CMV-p35 increased 52.01±2.90% inhibition of apoptosis compared with Ac-CMV-eGFP. The efficiency of apoptosis inhibition increased as the MOI increased. The highest percentage of inhibition of apoptosis by using 100 MOI Ac-CMV-p35 was 83.00±0.57% (Fig. 8). This result indicated that the expression of p35 mediated by recombinant baculo-virus vectors was capable of inhibit apoptosis induced by UV efficiently.
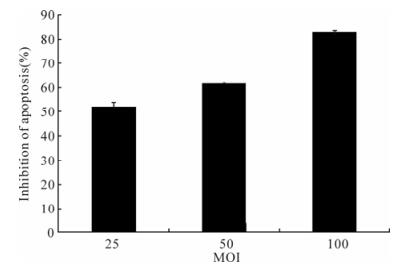
Figure 8. MOI of baculovirus-mediated p35 gene expression and resistance of 293 cells apoptosis induced by UV. Cells were transducted with Ac-CMV-GFP and Ac-CMV-p35 at 25, 50, 100 MOI for 2 h, and cells were induced by UV, after 36 h post-transduction. Percentages of apoptosis cells were detected by Hochest 33258 staining after 54 h. Percentages of apoptosis cells were detected by Hoechst 33258 staining after 54 h. To calculate relative inhibition, apoptosis cells of duplicate samples were compared to Ac-CMV-GFP and were multiplied by 100.
-
We further analyzed the possible effect of baculovirus-mediated expression of p35 gene on serum starvation-induced apoptosis. The results have clearly demonstrated that Ac-CMV-p35 inhibits serum starvation-induced apoptosis (Fig. 9). Treatment with starvation by adding serum free medium caused only 19.07±2.14% survival rate in control 293 cells, but 74.96±6.29% in 293 cells transduced by baculovirus Ac-CMV-p35. Anti-starvation ability increased with increasing MOI of Ac-CMV-p35. This result demon-strated baculovirus-mediated expression of p35 gene protected 293 cells from starvation efficiently.
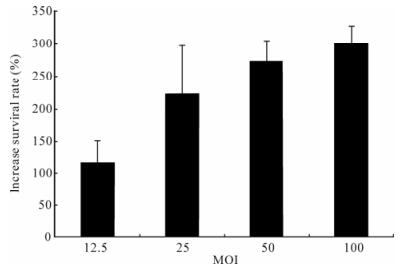
Figure 9. MOI of baculovirus-mediated p35 gene expression and resistance of 293 cells starvation. Cells were transducted with Ac-CMV-GFP and Ac-CMV-p35 at 12.5, 25, 50, 100 MOI for 2 h, and cells were cultured by DMEM free serum for 9 d. To calculate relative inhibition, numbers of trypan blue negative cells of duplicate samples were compared to Ac-CMV-GFP and were multiplied by 100.
Construction of recombinant baculovirus
Recombinant baculovirus-mediated GFP gene expression
Baculovirus-mediated p35 gene transfer into 293 cells
Cell viability assay and cell curve
Antiapoptosis induced by Actinomycin D
Antiapoptosis induced by UV
Resistance to starvation
-
The mechanism of P35 preventing cell apoptosis is thought to be the inactivation of caspases, inhibiting mitochondrial cytochrome C release and reduction of cellular reactive oxygen species (16, 20, 21). In this study, we found that baculovirus-mediated expression of p35 inhibited human embryo kidney 293 cells apoptosis induced by Actinomycin D and UV. As cell death inducers, Actinomycin D induces apoptosis via inhibiting RNA synthesis while UV induces apoptosis via damaging DNA strands and increasing production of P53 protein (9, 18). The most possible common pathway shared by these two cell death inducers in inducing apoptosis might be activation of caspases. It might be inactivation of caspases through which expressing of p35 prevented cell apoptosis in response to Actinomycin D and UV. However, expression of p35 may also prevent cell death via different mechanisms to break the apoptosis pathways of these two apoptosis inducers respectively.
Cellular proliferation is one important basic phy-siologic function, and we evaluated the effect of baculovirus-mediated expression p35 on cellular proliferation with different assays in this study. Baculovirus-mediated expression p35 had no adverse effect on proliferation as measured by MTT and cell growth curve assay. The p35-expressing and non-expressing cells had similar proliferation rates. These results eliminated the possibility of the cellular transformation and cancerization by baculovirus-mediated expression p35. In addition, these data more clearly attributed the effect of cell death resistance of baculovirus-mediated expression p35 to anti-apoptosis function of p35 rather than promoting cell pro-liferation function of p35.
The apoptosis-related mechanisms contribute to several pathologic processes of kidney diseases such as inflammation and acute renal failure (3, 13, 22). In experimental models of renal injury, apoptosis contribute significantly to cell death in response to hypoxia and ischemia. Inflammation is known to play an important role in exacerbating parachymal damage during reperfusion. With experimental models resear-chers also demonstrated renal tubular cell apoptosis was a major pathologic factor for ischemic and toxic acute renal failure. The administration of caspase inhibitors ameliorates renal inflammation and acute renal failure (5, 6, 9, 24). All these studies provide solid evidence that cell apoptosis is an important contributor to the pathogenesis of acute renal failure or ischemic injury. However, non organspecific administration of caspase inhibitors will lead to some side effects since these inhibitors inhibit the caspases systemically required for apoptosis as well as those necessary for cytokine activation. In this study, we demonstrated that baculovirus-mediated expression of the p35 gene could effectively prevent human kidney cell apoptosis and enhance cell viability. The transduction efficiency of baculovirus vectors in kidney cells was high, and high expressional level of target genes could be achieved. In addition, with cellular proliferation assay we demonstrated bacu-lovirus vectors did not have the ability to promote kidney cell tumorgenesis. Importantly, using a tissue-specific promoter in the context of baculovirus vectors will provide cell type-specific transgene expression in kidney cells. Experiments to verify this have been planned and will be conducted. All these features of the baculovirus vector offer it practical implications for gene therapy in the apoptosis-related kidney diseases as well as apoptosis related diseases in other organs.














 DownLoad:
DownLoad: