-
An estimated 8.6 (6.0-13.0) million people are living with HIV in Asia in 2006: 6.0 million men; 2.4 million women; ~200 000 children. In 1006 alone, there were 960 000 (640 000-900 000) new infections and 630 000 (430 000-900 000) AIDS death (29). The infection rates are still low in Asia, compared with some other continents, particularly Africa. However, since the populations of many Asian countries are so large, even low HIV prevalence rates means large numbers of people are living with HIV.
While HIV/AIDS epidemics come later in Asia, Asia is becoming an epicenter of 2nd largest epidemic after sub-Safaran Africa. In contrast to sub-Saharan Africa where the heterosexual transmission is a major driving force of the epidemic, the epidemic in Asia shows much complex structure. One third of HIV infections are acquired through injecting drug use in Asia. In addition to the epidemic through heterosexual route, the previously neglected epidemic among the men sex with men (MSM) has emerged in Asia (31). In the industrial countries in Asia, including Japan, Korea, Singapore, China Taiwan and Hongkong, MSM is the most important risk group and also significant numbers of HIV infection occurred among hemophiliacs by contaminated blood products in early 1980s. Molecular epidemiology has been a useful tool to analyze the origin of HIVs and to track a course of global HIV spread, providing the in-depth knowledge on AIDS epidemic: The study areas include the analyses of the distribution of HIV genotypes in different geographic areas, route of virus spread and specific association of genotypes with different epide-miologic features, such as risk behaviors. In this article, we overview the current status of HIV/AIDS epidemic and the recent advance in the study on the molecular epidemiology of HIV in Asia.
HTML
-
The Asia-Pacific region is vast and diverse and HIV epidemics in the region share that diversity. Table 1 summarizes the UNAIDS/WHO HIV estimates in different regions in Asia (29). We describe the outline of the epidemics in selected countries in Asia.

Table 1. HIV projection in various countries in Asia (end 2005) (UNAIDS/WHO)
-
In India, the world's second most populous country, an estimated 5.7 million (The world's largest) were living with HIV in 2006. While the heterosexual transmission is the predominant mode (more than 80%) of HIV infections in most of regions in India, the injecting drug use is the main driver of the HIV epidemics in the northeast, especially in the state of Manipur and Nagaland, near the border with Myanmar. There is a substantial overlap between injecting drug use and paid sex in some areas of the country. In Tamil Nadu, HIV prevalence among sex workers is 50%.
-
In China, an estimated 650 000 were living with HIV/AIDS at the end of 2005. The highest numbers of HIV infection have been reported from Yunnan (southwest), Henan (East Central), Xinjiang (Northwest), Guangxi and Guangdong (Southeast) provinces. HIV epidemic began in rural areas before spreading to cities-unusual pattern of HIV spread. Injecting drug use accounts for nearly half (44%) of the people living with HIV. However, a half of the new HIV infection occurred during unprotected sex. As HIV is spreading gradually to the general population, the numbers of HIV infection among women are growing. The unique features of epidemic in China is that a substantial portion of infections were caused by unhygienic practice of commercial plasma collection in central China during the early 1990s. An estimated 250 000 people, mainly rural peasants, were infected in the provinces, including Henan, Anhui, Shanxi, Hubei and Shngdong. Recent study found the evidence of sexual transmission of HIV to non-donors. HIV epidemic starts to go beyond high risk groups into general population.
-
In Thailand, an estimated 580 000 were living with HIV/AIDS with national adult HIV prevalence of 1.4% at the end of 2005. Because of intensive prevention measures, the new annual infection rate continues to drop: the estimated 18 000 new infections in 2005 from the peak incidence of 140 000 in early 1990s. A large proportion of new HIV infection is in general population. Recent studies show that more than one-third of HIV infections in 2005 were among women who had been infected by their long-term partners. Moreover, the previously neglected epidemic among MSMs has been recognized relatively recently. HIV prevalence among MSMs in Bangkok has increased steeply from 17% in 2003 to 28% in 2005.
-
An estimated 360 000 were living with HIV/AIDS at the end of 2005 with national prevalence of 1.3%. HIV prevalence among young people (15-24 years of age) was at concerned level of 2.2%. The high HIV infection levels were found in the high risk groups: 43% in IDUs and 32% among sex workers. However, there are some indications that the epidemic might be slowing down: National prevalence among pregnant women has declined from 2.2% in 2000 to 1.3% in 2005.
-
An estimated 260 000 are living with HIV at the end of 2005, more than doubled since 2000. Appro-ximately 40 000 people are newly infected with HIV each year. Injecting drug use and sex work are the main factors driving the epidemic. HIV prevalence among IDUs increased from 9% in 1996 to 32% in 2003. The levels of infection are reaching as high as 63% (in Hanoi) and 67% (in Hai Phong) in 2005. Up to 12% of sex workers and 18% of the sexual partners of IDUs were found to be infected with HIV, and the prevalence among pregnant women reached 1% mark in some regions.
-
An estimated 20 000 were living with HIV/AIDS in Japan at the end of 2005. More than 60% of new infections are among MSMs and 25% are acquired through heterosexual contacts. One out three new HIV/AIDS case reports is from AIDS patients, suggesting that a significant proportion of HIV-infected persons do not know their infections and visit clinics in the very late stage of diseases.
India
China
Thailand
Myanmar
Vietnam
Japan
-
Human immunodeficiency virus (HIV) are divided into two related human retroviruses, HIV-1 (HIV type 1) and HIV-2 (HIV type 2) (Fig. 1). HIV-1 is distributed worldwide, while HIV-2 is confined primarily to West Africa. Outside West Africa, a significant number of HIV-2 cases have been reported form southern/western India (6, 20) and Korea (9, 17).
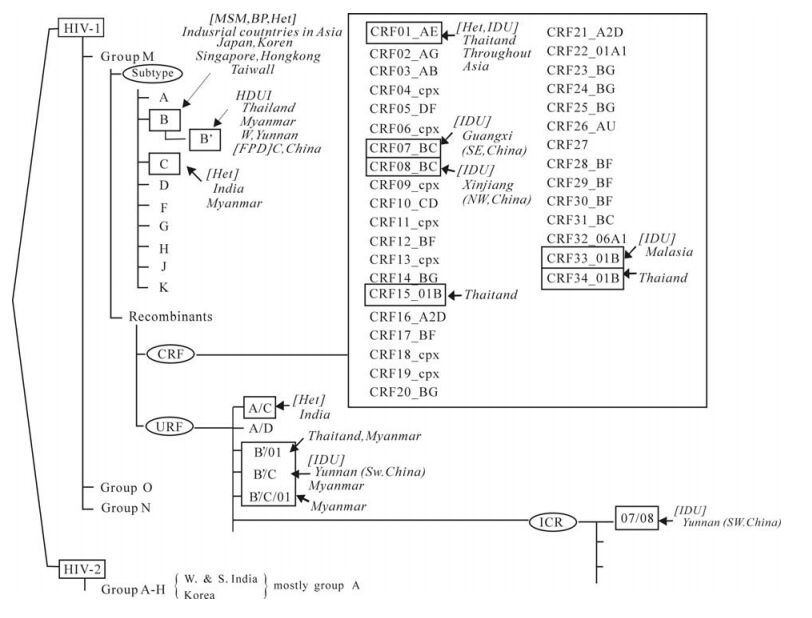
Figure 1. Classification of HIV genotypes and their origins. HIVs are classified into types (types 1 and 2), groups (M, O and N for HIV-1; A through G for HIV-2), subtypes (A through K for HIV-1) and subsubtypes (A1 and A2, F1 and F2). HIV-1 recombinants are categorized into circulating recombinant forms (CRFs) and unique recombinant forms (URFs). HIV-1 subtype B' (B "prime") (Thailand variant of subtype B, also referred to as Thai-B), a unique regional subtype variant strongly associated with bloodborne transmission (prevalent among IDUs and former plasma donors/paid blood donors) in Asia (see text).
HIV-1 strains were classified into three distinct groups of HIV-1 (M, N, and O) (Fig. 1). The vast majority (more than 95%) of HIV-1 strains belong to group M (for Major or Main). Group O (for Outlier) infections are limited to people living in Central Africa (Cameroon, Gabon and Equatorial Guinea), but even in this area they represent a small minority of HIV-1 infections (3, 7, 13, 30). Only a few cases of group N (for New, or non-M/non-O) infections were identified in only limited numbers of patients from Cameroon (23). Groups O and N viruses were not detected in Asia.
Pandemic HIV-1 group M viruses are classified into at least 9 discrete genetic subtypes (A-D, F-H, J, K) based on the sequence of complete viral genomes (Fig. 1). In some subtypes, subclusters within subtypes were identified, leading to a classification into subsubtypes: subsubtypes A1 and A2 within the subtype F; subsubtypes F1 and F2 within subtype F.
-
It was realized that certain HIV-1 strains clustered with different subtypes in different regions of their genomes. Some of these mosaic HIV-1 genomes have been identified in several, apparently unlinked, individuals and play a major role in the global AIDS pandemic, designated as "circulating recombinant forms", or CRFs (2). A total of 34 CRFs are currently recognized (http://hiv-web.lanl.gov/CRFs/CRFs.html) (Fig. 1). Six CRFs, CRF01_AE (former subtype E) (original site of isolation: Thailand), CRF07_BC (Xinjiang, China) and CRF08_BC (Guangxi, China), CRF15_01B (Thailand), CRF33_01B (Malaysia) and CRF34_01B (Thailand) have been identified so far in Asia (Fig. 1 and Fig. 2).
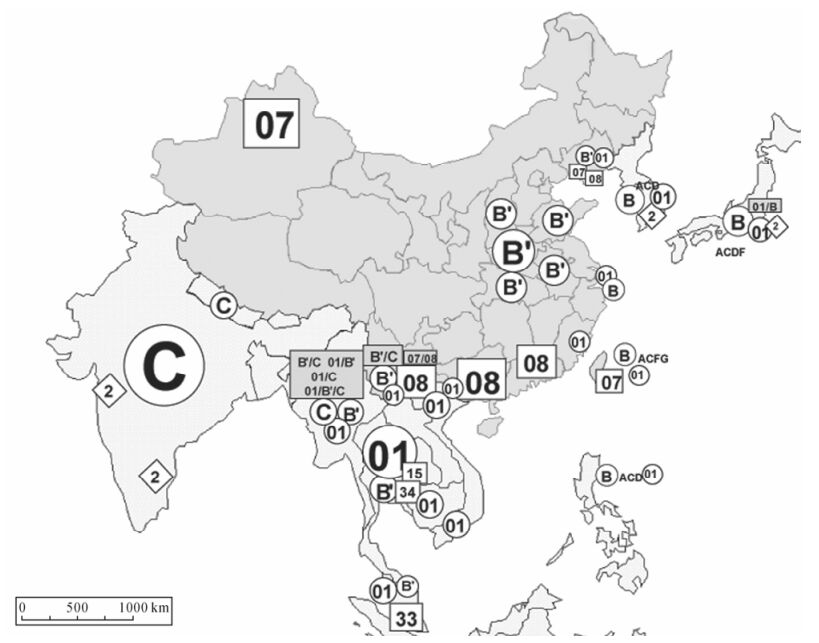
Figure 2. Geographical distribution of HIV genotypes in Asia. The distributions of HIV genotypes (types, subtypes/subsubtypes and CRFs/URFs) in Asia are shown. '2' in rhombus, HIV-2; 01, CRF01_AE; 07, CRF07_BC, 08, CRF08_BC, 15, CRF15_01B; 33, CRF33_01B and 34, CRF34_01B _34. Various URFs comprised of the indicated genotype are shown in the shodowed box.
-
In addition to CRFs, various types of 'unique' recombinant forms (URFs) have been reported, currently without evidence of epidemic spread (Fig. 1) (14). URFs are diverse forms of HIV-1 intergenotype recombinants with unique mosaic structures, seen only in a single person or in a few epidemiologically-linked individuals. Most of URFs have been detected in the regions where multiple subtypes are co-circulating (Fig. 2). A various types of URFs were detected in several locales in Asia: the intersubtype A/C recombinants in India (12), CRF01_AE/B recombinants in Thailand (14); diverse recombinant forms comprised of various combination between subtypes B and C and CRF01_AE in Myanmar (15, 16, 26); the intersubtype B/C recombinants in Yunnan Province, China (34, 35) (Fig. 2). The detection of substantial numbers of different URFs worldwide suggests that dual/mutiple infections or super-infections with different lineages of HIV-1 strains might not be a rare event.
-
On a global scale, the most prevalent HIV-1 genotypes are subtypes C (56%), followed by A (23%), B (8%), D (5%) and CRF01_AE (5%) in 1999 (5). In Asia, HIV-1 subtypes B, US-European strain, is found among MSMs, hemophiliacs and some of hetero-sexual cases in the industrial countries in Asia, including Japan, Korea, Singapore, China Taiwan and Hongkong (Fig. 2 and Fig. 3). HIV-1 subtype C predominates in India (12). HIV-1 CRF01_AE is
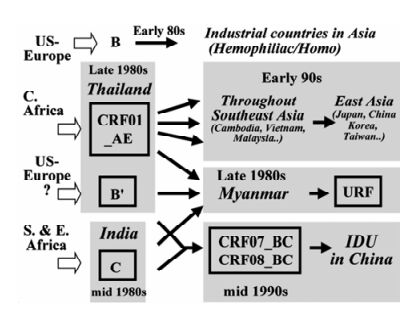
widely circulating in Southeast Asia (18, 19, 32). CRF01_AE epidemic broke out in the late 1980s among female commercial workers and their clients in Thailand (19, 33) and became prevalent even among injecting drug users (IDUs) in this country. CRF01_AE quickly started disseminating throughout Southeast Asia, including Cambodia, Vietnam, Malyasia, China, China Taiwan, Korea and Japan (Fig. 3) (32, 33). Subtype B' (Thailand variant of subtype B; also referred to as Thai-B virus) (8, 18, 19) is a unique subtype B variant that spread primarily though IDU networks in southeast Asia (16, 33) and is the founder strain causing outbreaks among former plasma doors in Central China (24) (Fig. 2). Two closely-related CRFs, CRF07_BC and CRF08_BC are disseminating rapidly among IDUs in northwestern (Xinjiang Province) (25) and southeastern (Guangxi Province) China (21), respectively. A variety of novel CRFs comprised of CRF01_AE and subtype B (B') have been recently identified in Thailand (CRF15_01B (28) and CRF34_01B) and Malaysia (CRF33_01B) (27) (Fig. 1 and Fig. 2).
-
Recombinant viruses have already contributed substantially to the global pandemic as well as in Asia. We found that substantial proportions of HIV-1 strains were the URFs in Western Yunnan (65%) (34) and Central Myanmar (10%-30%) Myanmar (11, 15, 16, 26) (Fig. 2). Even recombinant viruses will recombine, leading to evolution of second-generation recom-binants, inter-CRF recombinants (ICRs): ICR01_0708 identified among IDUs in southwesten China (Yunnan Province) is the first example of this category, which is comprised of two closely related CRFs in China, CRF07_BC and CRF08_BC (35).
-
HIV-2s are known to be members of a broader HIV-2/Simian immunodeficiency virus (SIVsm) phy-logenetic group. HIV/SIVsm phylogenetic groups are classified into eight genetic "groups" (A through H) (4). However, only HIV-2 groups A and B are disseminated into significant numbers of human popu-lations (1, 22).
In Asia, HIV-2 infections were reported in west and south India, frequently associated with dual infections of subtype C strains (6, 20). Outside India, 10 cases of HIV-2 infections have been reported in Korea (17) (Fig. 1). While most of the HIV-2 infection cases in Asia were the infections with HIV-2 group A, one HIV-2 group B infection was identified in a person with Korean nationality living in Japan (10), who appears to be infected through heterosexual contacts in DR Congo (17).
Circulating recombinant form (CRF)
Unique recombinant form (URF)
Distribution of HIV-1 genotypes in Asia
Geographical recombination hot spots in Asia
Genotype classification and geographic distri-bution of HIV-2
-
Molecular epidemiology of HIV provides the basis of understanding the origins and genesis of the epidemics in different regions in Asia and their relationship, particularly elucidating the pathway of viral spread over the regions and risk populations. Studies also provide information to delineate the mechanism of person-to-person transmission and viral evolution. However, the biological significance of the global diversity of HIV-1 strains remains to be defined. For instance, the relationship between HIV-1 genotype and biological phenotypes is not well established. In-depth knowledge obtained from the study on molecular epidemiology of HIV is critically important to elucidate the dynamics of HIV spread and to formulate future vaccine and other prevention strategies in Asia.














 DownLoad:
DownLoad: