-
The liver is particularly susceptible to TNFα-mediated cytotoxicity[7]. TNFα is a pleiotropic cytokine that can produce multifold effects such as cell proliferation, differentiation, survival or death and is involved in a variety of physiological and pathological processes[9]. Two different receptors, p55 TNFR1 (TNFRSF1A) and p75 TNFR2 (TNFRSF1B), transduce the TNFα signal. TNFR1 is a death receptor, and also transduces survival signals [3, 5]. RNA interference is an evolutio-nally conserved gene silencing mechanism present in a variety of eukaryotic species, and has been proven to be an extremely potent and versatile tool to specifically reduce expression of targeted genes [1, 12]. RNAi uses short double-stranded RNA to trigger degradation or translation repression of homologous RNA targets in a sequence-specific manner [6].
HTML
-
MHV-3 was obtained from the American Type Culture Collection (ATCC, Manassas, VA), plaque purified on monolayers of DBT (delayed brain tumor) cells, and titered on L2 cells according to a standard plaque assay.
-
Female BALB/cJ mice, 6-8 weeks of age and weighing 18-20 g, were purchased from Hubei Provincial Institute of Science and Technology (Wuhan, China). Gene transfection was achieved by hydrodynamic injection, via the tail vein, of 100 g of mTNFR1 shRNA plasmid (positive group) or control plasmid (control group), dissolved in 2 mL of normal saline over 4 to 5 sec (18 animals in each group). The injection was repeated 24 h later, and 24 h later mice received 20 plaque-forming units (PFU) of MHV-3 intraperitoneally to develop fulminant viral hepatitis.
-
Mouse U6 promoter(U6P) was cloned into pMSCV vector. The shRNA template was amplified from pMSCV-U6 expression vector by PCR. By the transition of pMD-18 T-vector, purified shRNA frangment DNA were subcloned into pMSCV vector (Invitrogen, USA) at the BamH I and Hind Ⅲ restriction sites to construct the plasmid named p-mTNFR1shRNA. By using the same vector, the same long fragment of irrelevant shRNA plasmid was also constructed as an experiment control. The sequence for mTNFR1 shRNA plasmid was verified correct.
-
Total RNA was extracted from mouse liver using TRIzol reagent (Invitrogen, USA) and reverse trans-cribed into cDNA (Fermentas, USA) according to the manufacturer's standard protocol. Real-time fluorescence quantitative RT-PCR was done with TOYOBO PCR Mix according to the manufacturer's standard protocol to detect mTNFR1 expression at the mRNA level. The upstream primer was 5'-GGTCTTTGCCTTCTATCC TT-3', and the downstream primer was 5'-TTCCAG CCTTCTCCTCTTT -3' for mTNFR1 gene. GAPDH was used as an internal reference.
-
Immunohistochemical staining was performed with a rabbit polyclonal antibody against mTNFR1 (Abzoom, USA). Cultured slices were blocked with 10% normal goat serum in PBS at room temperature for 2h. The slices were incubated with Ab (20μg/mL in PBS) at room temperature for 2h. Subsequently, slices were incubated with immunoperoxidase-con-jugated goat IgG fraction to rabbit IgG Fc at room temperature for 1h, following which they were washed five times in PBS with 0.05% Tween20. Cultured slices were then air dried and photographed with a microscope.
-
Quantitative data were expressed as means±SD. Statistical analysis was carried out by one way analysis of variance, and a P < 0.05 was considered statistically significant.
Virus
Animals
Construction of mTNFR1 shRNA plasmids
Real-time fluorescence quantitative RT-PCR
Immunohistochemical staining
Statistical analysis
-
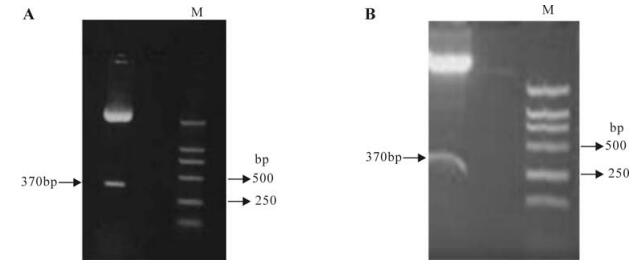
The mTNFR1 shRNA plasmids were successfully constructed as evidenced by the restriction enzyme mapping shown in Fig. 1 and further confirmed by sequence analysis. The plasmids named p-mTNFR1-shRNA complimentary to the sequence responsible for the functional key point were constructed.
-
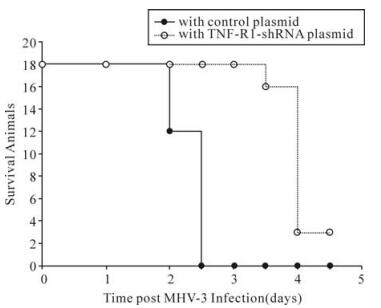
To further investigate the biological effect of mTNFR1shRNA plasmid in vivo, shRNA plasmid was delivered twice by hydrodynamic injection into 18 MHV-3-infected BALB/cJ mice. In the mTNFR1 shRNA inteference group, all 18 mice with shRNA plasmid were alive on day 3 postinfection, and 2 of 18 mice (13.3%) recovered from fulminant viral hepatitis (Fig. 2). In contrast, no mice in the control plasmid-treated group survived to day 3.
-
Liver histology was examined by hematoxylin and eosin (H & E) staining at the indicated times. There were rare or noinfiltrating inflammatory cells, and hepatocyte necrosis in p-mTNFR1shRNA inteferenced mice at 24, 48, 60 h after MHV-3 infection (Fig. 3) However, mild to moderate inflammatory cell in-filtration and spotty necrosis were evident in the hepatic lobules of control plasmid treated mice. These data strongly suggested that the mTNFR1shRNA plasmid improved the pathology in MHV-3-induced fulminant viral hepatitis; in some cases the pathological process was reversed, allowing the animals to recover from this disease.
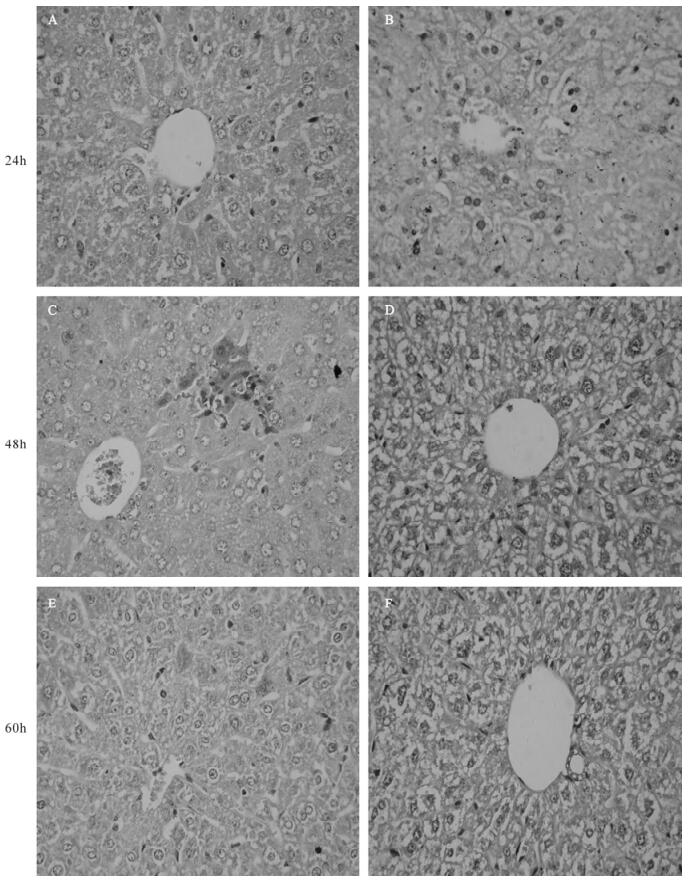
Figure 3. Effect of mTNFR1 shRNA plasmid on liver pathology in MHV-3 infected BALB/cJ mice. Livers were collected from control plasmid treated (A, C, and E) and mTNFR1shRNA plasmids treated (B, D, and F) BALB/cJ mice 24 h (A and B), 48 h (C and D), and 60 h (E and F) after MHV-3 infection and pathologic characteristics were investigated by H & E staining.
-
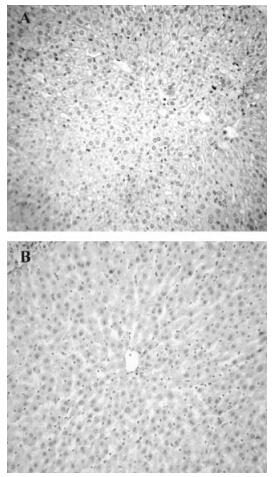
To evaluate the inhibitory effects of mTNFR1-shRNA on mTNFR1 expression in vivo, a time course study of the effects was performed by real-time fluorescence quantitative RT-PCR, using liver samples from MHV-3-infected mice 24, 48 and 60 h postinfection. It was evident that the inhibitory effect of shRNA plasmids began 24 h postinjection (Fig. 4). These data were further confirmed by immuno-histochemistry, using a specific polyclonal antibody against mTNFR1 (Fig. 5). Results indicated none or rare mTNFR1 expression in the liver, with rare or mild inflammation in shRNA plasmid treated mice compared with control plasmid treated mice.
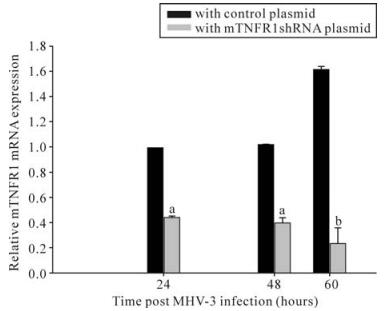
Figure 4. Livers from mTNFR1shRNA plasmid treated and control plasmid treated BALB/cJ mice were collected 24, 48 and 60 h after MHV-3 infection, determined by quantitative real-time PCR. In the mTNFR1shRNA treated mice liver, mTNFR1 gene expression were remarkably reduced compared with the control plasmid treated mice. aP < 0.01, bP < 0.05.
Construction of mfgl2 and mTNFR1 shRNA plasmids
Elevate the survival rate of BALB/cJ mice with fulminant hepatitis
Inteference of mTNFR1shRNA plasmid improved murine liver pathology
Effect of shRNA plasmids on mTNFR1 expression in vivo
-
Worldwide about 400 million people are chronically infected with the hepatitis B virus (HBV), especially in Asia, where there is an HBV chronic carrier rate of 10%-15%. In the Far East, fulminant hepatic failure is mainly due to viral hepatitis. The mortality of fulminant viral hepatitis is over 80% in situations where there is alack of immediate liver transplantation [4]. Fulminant hepatitis is characterized by recurrent flares of hepatocellular injury, but the mechanisms for virus-induced hepatocyte injury are still not clearly known.
The liver is particularly susceptible to TNFα-mediated cytotoxicity. TNF-induced apoptotic death of hepatocytes may also be of major importance under conditions causing acute liver failure in septic shock. Previous studies suggested that the TNFR1 is key candidate for target interference to prevent cell death. The TNFR1 molecule has been reported to induce apoptosis and massive tissue destruction in murine liver. In the liver, massive apoptosis is thought to be involved in hepatocyte homeostasis and to play important roles in a variety of diseases [10]. In fact, hepatic failure and tissue destruction as a result of endogenously produced TNF has recently been demonstrated to be mediated by the 55-kDa TNF receptor (TNFR1), which is structurally related to the fus/Apo-1 Ag [11]. Hepatocytes apoptosis occurred in the liver plays an important role in the process of many liver diseases, especially hepatic failure caused by various reasons. Actually LPS toxicity is due to serious apoptosis that further induce liver injury and destruction in the FHF model induced by LPS/D-GalN. However the contribution of hepatocytes apoptosis to the development of murine hepatitis virus strain 3 (MHV-3) induced hepatic failure in Balb/cJ mice has never been explored, which in turn might be one of the important aspects for understanding the pathogenesis of hepatocytes injury.
Studies from ours and many others have shown that TNFα-mediated hepatocyte apoptosis have been implicated in a wide range of liver diseases including viral hepatitis, alcoholic hepatitis, ischemia/ reperfusion injury, fulminant hepatic failure and cancer. Experi-mental evidence in animal models indicates that the coagulation cascade and TNFR1-mediate apoptosis play a crucial role in the outcome of inflammatory insults. In this study, We have recently successfully constructed the mTNFR1 shRNA plasmid[12]. Gene transfer of mTNFR1 shRNA plasmid in vivo also resulted in a significant reduction of mTNFR1 expression and improvement of liver function and histology, prolongation of the survival time period, and elevation of the survival rate in BALB/cJ mice with MHV-3-induced fulminant hepatitis[14].
There has been significant interest in the use of RNA interference (RNAi) inhibition of gene expression[1, 12]. RNAi is thought to catalytically trigger degradation of target mRNAs before translation. It is therefore possible that RNAi could act synergistically to specifically result in more efficient gene silencing[6, 13, 17]. Song et al used RNA interference targeting Fas to elevate the survival rate in mice with fulminant hepatitis and protect mice from fulminant hepatitis[14]. we have recently demonstrated that a novel specific shRNA plasmids can be successfully used to block the expression of mTNFR1 in a cell culture system. Here we further showed that the shRNA expression plasmid ameliorates liver pathology including inflammatory infiltration, and hepatocyte apoptosis in animal model. Furthermore, it prolonged the survival period and elevated the survival rate of MHV-3-infected BALB/cJ mice, which normally died of a fatal disease, fulminant hepatitis[16]. This study may provide an effective therapeutic strategy for a targeting intervention in the disease control to which the gene TNFRI contributed.













 DownLoad:
DownLoad: