-
Interferon stimulated gene 15 protein (ISG15) is one of the ubiquitin-like modifiers whose expression can be robustly induced by type Ⅰ interferon [4]. Although ISG15 has been identified for more than 30 years [5], its function have only recently been determined [3]. ISG15 and its modification function play roles in many processes including cellular signal regulation, pregnancy preparation and anti-viral functions [3]. Moreover, mice deficient in ISG15 were more susceptible to influenza, herpes, and Sindbis viruses' infection [13]. ISG15 can repress the replication of HIV-1 or Ebola virus by inhibiting the release of virions [20, 21].
Bovine ISG15 (bISG15), which has 70% homology amino acid sequence to human ISG15 [23], is up-regulated in the endometrium in response to conceptus-secreted IFN-τ during early pregnancy [6]. Up to now, most studies have focused on the role of bISG15 in reproductive biology, little is known about its role in innate immune function. We found the expression of bISG15 in fetal bovine lung (FBL) cells could be induced after treatment with Poly Ⅰ:C or lipopolysaccaride (LPS) [14]. Furthermore, bISG15 expression can also be induced in bovine immunode ficiency virus (BIV) infected FBL cells, and the over-expression of bISG15 can repress the replication of BIV in FBL cells [15]. Therefore, bISG15 can be regarded as an antiviral and inducible protein in FBL cells infected with BIV. Besides our work, it has been reported that the expression of bISG15 in maternal blood is increased in cows infected with acute non-cytopathic bovine viral diarrhea virus (BVDV) [26].
Bovine herpesvirus type 1 (BHV-1) is a significant viral pathogen of cattle that can lead to respiratory and genital disorders, abortion, conjunctivitis, and multisystemic infection [10, 11]. Interferon signaling plays an important role in the process of herpes virus infection. Infection of cultured human cells with herpes simplex virus type 1 (HSV-1) can lead to production and secretion of interferon (IFN) [27]. After viral infection, the expression of some viral genes, such as ICP0 / ICP34.5 / Us11, can inhibit IFN and gene expression induced by IFN [22, 25, 28]. IFN also plays an important role in controlling BHV-1 replication and pathogenesis. It has been reported that mice lacking type Ⅰ and type Ⅱ interferon receptors die within a few days following BHV-1 infection, however BHV-1 infection of WT mice does not lead to clinical symptoms [2].
In our previous work, we have demonstrated that Poly Ⅰ:C can stimulate the expression of bISG15 robustly in FBL cells and bIRF-3 plays an important role in this process [14]. Considering the cascade relation among bISG15, the IFN pathway and BHV, in this study, we detected the transcription of bISG15 in FBL cells infected with BHV-1 and tried to find which viral gene or protein might take part in this process.
HTML
-
MDBK(Madin-Darby Bovine Kidney) cells, 293T cells and FBL cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS, 1% penicillin-streptomycin in 5% CO2 at 37℃. BHV-1was provided by Dr. Charles Wood (University of Nebraska Lincoln) and was cultured with MDBK. BHV-1 virus stock was added onto FBL cells with a multiplicity of infection (MOI) of 0.01.
-
Poly Ⅰ:C and CHX (cyclonheximide) were purchased from Sigma-Aldrich. Poly Ⅰ:C was dissolved in PBS at a concentration of 2.5 mg/mL. CHX was dissolved in dimethyl sulfoxide at a concentration of 100 mg/mL. The luciferase reporter plasmid pGL3-bISG15p was constructed by inserting a region of bISG15 promoter (-363 to +19) into a pGL3-basic vector. The bIRF-3 expression plasmid pcDNA-bIRF-3 was constructed by inserting the full length bIRF-3 gene into a pcDNA3.1(+) vector. The bICP0 expression plasmid pcDNA-bICP0 was constructed by inserting the full length bICP0 gene into a pcDNA3.1(+) vector.
-
Total RNA was isolated with TRIzol reagent (Invitrogen) from cultured cells. RNA samples were treated with DNase I (Takara) and quantified using BioPhotometer (Eppendorf). 1 μg RNA was reverse transcribed into cDNA using 5 μL 5 × reaction buffer, 0.2 mmol/L dNTPs, 0.8 μmol/L oligo-dT, 0.5U RNase inhibitor and 4 U Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Promega) in 25 μL reaction system at 42℃ for 1 h.
-
Real time PCR was performed using an IQ5 Multicolor Real-time PCR detection system (Bio-Rad). The primers used to quantify bISG15 and GAPDH (served as endogenous control) were bISG15-F (5'-GTGGTGCAGAACTGCATCTC-3'), bISG15-R (5'-GCCAGAACTGGTCTGCTTGT-3'), GAPDH-F (5'-AACGGCACAGTCAAGGCAGA-3'), GAPDH-R (5'-TCGGCAGAAGGTGCAGACAT-3'). Each PCR system contained 0.5 μL EVA Green nucleic acid stain (Biotium) to detect fluorescence. Cycling parameters were 94℃ for 3 min, followed by 40 cycles at 94℃ for 30 s, 56℃ for 30 s and 72℃ for 30 s. Fluorescence measurements were taken at each cycle during the step of 72℃. The level of bISG15 expression was normalized to GAPDH using the 2(-△△Ct) method [16].
-
293T cells were passaged in 24-well plates and cultured at 37 ℃ for 12 h. Then, these cells were co-transfected with reporter plasmid (pGL3-bISG15p) and expression plasmids (pcDNA-bIRF-3, pcDNA-bICP0).pCMVβ-gal was used as the control for transfection efficiency. Total amount of DNA were equalized by adding the vector DNA (pcDNA3.1). At 48 h post-transfection, cells were harvested and luciferase assays were performed (Promega). The transfection efficiency was assessed by determining the β-galactosidase activity.
Cells and viruses
Materials and plasmids
RNA isolation and reverse transcription
Real-time PCR assay
Luciferase assay
-
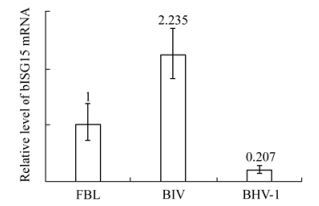
To detect whether BHV-1 infection has an effect on the basal expression of bISG15, cell free BHV-1 was added into the FBL cells. FBL cells infected with BIV were taken as a positive control, as we knew that BIV infection could induce the expression of bISG15 in FBL cells [15]. Uninfected FBL cells were taken as a negative control and normalized as 1. It was shown that there appeared to be no obvious cytopathic effect in FBL cells infected with BHV-1 or BIV. After 12 h of viral infection, cells were harvested and real-time PCR assay was performed. As Fig. 1 shows, BHV-1 infection decreased the basal mRNA level of bISG15 in FBL cells. In contrast, BIV infection increased the mRNA level of bISG15 in FBL cells. The basal transcription of bISG15 was repressed in FBL cells infected with BHV-1.
-
As we knew BHV infection inhibited the basal expression of bISG15 in FBL cells, we were trying to address whether BHV-1 infection repressed the inducible expression of bISG15 in FBL cells treated with Poly Ⅰ:C. As shown in Fig. 2, there were 10 groups of FBL cells passed to bISG15 mRNA translation assay using RT-PCR. FBL cells untreated and uninfected were taken as negative control and normalized as 1. The groups treated with Poly Ⅰ:C were treated by adding Poly Ⅰ:C into the cell medium to a final concentration of 20 μg /mL. The groups treated with CHX were treated by adding CHX into the cell medium to a final concentration of 50 μg /mL. CHX is a kind of translation inhibitor which can inhibit newly synthesized protein. After 8 h of adding Poly Ⅰ:C or CHX, FBL cells were infected with BHV or BHV(UV) at a MOI of 0.01. BHV(UV) was the BHV treated with UV254 for 1 h to inactivate viral infection. After 12 h of infection, cells were harvested and the assay was performed. As shown in Fig. 2, BHV infection repressed the inducible expression of bISG15 treated with Poly Ⅰ:C, however BHV(UV) did not work just as BHV. There was no statistical significance among the last three groups which were FBL cells treated with both Poly Ⅰ:C and CHX either with infection or uninfection of BHV or BHV(UV). It implies that BHV infection is inhibited by expression of bISG15 in FBL cells treated with Poly Ⅰ:C and this in turn is dependent on the level of protein synthesis. Taken them together, BHV infection repressed the induction of bISG15 in FBL cells treated with Poly Ⅰ:C, and it depended on BHV viral infection and protein synthesizing.
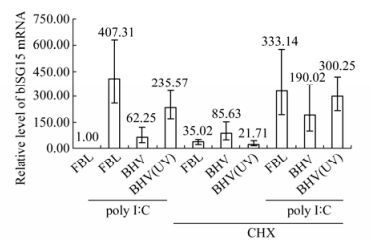
Figure 2. Real-time PCR assay of bISG15 stimulated transcription in FBL cells. FBL cells were treated with Poly Ⅰ:C to stimulate the expression of bISG15, or treated with CHX to inhibit new protein synthesizing. After treatment, cells were infected with BHV or BHV(UV) which had not efficient viral infection. After 12 hours infection, cells were harvested and real-time PCR assay was performed. FBL cells untreated and uninfected was taken as negative control and normalized as 1.
-
Based on the results obtained above, it was hypothesized that a BHV viral protein might take part in repressing the expression of bISG15 in FBL cells. The viral protein bICP0 which encodes by the immediate early gene of BHV was the candidate protein for repressing expression. The induction of bISG15 in FBL cells treated with Poly Ⅰ:C is mainly stimulated by cellular transactivitor bIRF-3 [14]. 293T cells were co-transfected with a reporter plasmid pGL3-bISG15p, which has a bISG15 promoter at the upstream site of luciferase gene, bIRF-3 expressing plasmid pcDNA-bIRF-3 and bICP0 expressing plasmid pcDNA-bICP0. As shown in Fig. 3, bIRF-3 over-expression stimulated the promoter of bISG15. Furthermore, bICP0 over-expression repressed the stimulation and the effect was dose-dependent.
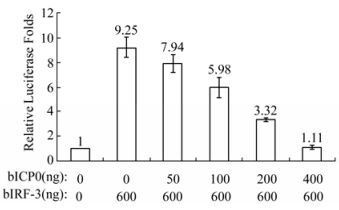
Figure 3. Luciferase expression of bISG15 promoter. 293T cells were co-transfected with bISG15 promoter luciferase plasmid pGL3-bISG15p, bIRF-3 expressing plasmid pcDNA-bIRF-3, bICP0 expressing plasmid pcDNA-bICP0. After cells co-transfected for 12 hours, cells were harvested and luciferase activity assay was performed. Cells co-transfected with pGL3-bISG15p and vector plasmid pcDNA3.1 were taken as negative control and normalized as 1.
BHV-1 infection repressed the basal transcription of bISG15 in FBL cells
BHV infection repressed the inducible transcrip tion of bISG15 in FBL cells treated with Poly Ⅰ:C
BHV protein bICP0 inhibited the activation of bISG15 promoter stimulated by bIRF-3
-
In this study, we detected the bISG15 transcription level in FBL cells infected with BHV-1. BHV infection could repress the basal expression of bISG15 or the induction of bISG15 stimulated by Poly Ⅰ:C. Furthermore, the repression was dependent on the BHV-1 viral infection and the synthesis of protein bICP0. It indicated some viral proteins might inhibit the expression of bISG15. The expression of ISG15 and enzymes involved in ISG15 modification are interferon inducible [4, 5, 9, 12, 19]. ISG15 and its modified form play important roles in innate immunity and antiviral response to infection, so it is reasonable that BHV-1 evolved via some mechanism to inhibit bISG15 and its modification system.
In our previous work it has been shown that bIRF-3 is the key factor in the induction of bISG15 in FBL cells treated with Poly Ⅰ:C [14]. IRF-3 commonly exists in the cytoplasm, after some stimulation by molecules such as dsRNA, then phosphorylated, dimerized and translocated to the nucleus to induce the expression of interferon stimulated genes (ISGs) [1]. BHV-1 viral protein bICP0 encoded as an immediate early (IE) gene is the first candidate which may repress the expression of bISG15. The bICP0 gene is the only BHV-1 gene known to inhibit interferon signaling [7]. Moreover, it has been reported that bICP0 targeted bIRF-3 for proteasome-dependent degradation [25]. Our results confirmed that bICP0 repressed activation of bISG15 promoter is stimulated by bIRF-3.
In conclusion, our studies showed that BHV-1 infection repressed both basal and inducible transcription of bISG15 in FBL cells and BHV-1 viral protein bICP0 may play a role in this process. There are still some questions to be answered, such as whether other BHV-1 viral patterns (Proteins and/or RNAs) take part in inhibiting expression, whether bICP0 is the key factor to repress the expression of bISG15 and what is the role of bISG15 in inhibiting BHV-1 infection. It has been reported that ISG15 could stabilize the existence of IRF-3 [17], thus, if IRF-3 is stimulated the by the expression of ISG15 [8, 14], then ICP0 can inhibit the activation of IRF-3 [18, 24]. It will be interesting to clarify the relationship among IRF-3, ISG15 and ICP0. Our work elucidating the functions of bICP0 and bISG15 provides a foundation for these future studies.













 DownLoad:
DownLoad: