-
Rabies is a zoonotic disease which is induced by the rabies virus and remains an important infectious disease worldwide, particularly in developing countries. Rabies can be prevented and controlled (for example, in China, about 12 million people receive rabies vaccination annually [6]) but about 55000 people still die of rabies every year in world, often as a consequence of inconsistent and untimely application of rabies post-exposure prophylaxis (PEP). The titer of anti-rabies neutralizing antibodies is an important parameter for evaluating the efficacy of the vaccine immunization in either the pre-exposure prophylaxis or PEP of rabies. To detect levels of anti-rabies neutralizing antibodies is also very important for clinical diagnosis of human rabies. The WHO recommends that booster immunizations should be carried out with reference to the titer of anti-rabies neutralizing antibody when it is lower than 0.5 IU/mL [1].
In October 2005, a WHO expert consultation on rabies recommended that evaluation of anti-rabies neutralizing antibody levels should be carried out using the rapid fluorescent focus inhibition test (RFFIT) or the fluorescent antibody virus neutralization test (FAVN) [1]. The RFFIT is also one of the standard methods specified in the Pharmacopoeia of the People's Republic of China (Chapter Three, 2010 edition) for use in the detection of anti-rabies neutralizing antibodies. In the RFFIT detection system, it is necessary to utilize a reference serum as a standard which is serial diluted, mixed with a standard quantity of live rabies virus and evaluated for its ability to neutralize the virus; the remaining virus in each dilution is then inoculated into cultured cells and checked with specific anti-rabies nucleoprotein antibody labelled with fluorescein (FITC anti-rabies monoclonal globulin). The quantity of the remaining virus is calculated according to the number of the foci of infections produced by remaining virus. The titer of anti-rabies neutralizing antibody in the samples is calculated according to the number of foci of infections and the quantity of standard serum, and expressed as International Units (IU)/mL. Therefore, the standard serum used as the reference in the RFFIT is a key element in the interpretation of RFFIT results.
Many different standard serums are used in RFFITs in different laboratories including: The WHO STD; the Chinese the NSSC STD (the standard serum in use in China which is prepared by human immunoglobulin with a titer of 21.4 IU/mL and titrated with reference to the WHO STD at a 30 IU/mL titer by the National Institute for Food and Drug Control of China) [2]; and the Office International des Epizooties (OIE) STD (OIE standard serum). When establishing RFFIT in our laboratory [4], we purchased WHO STD & NSSC STD and used both of them as standard serums. Besides observing a certain deviation between the labelled titer and the actual titer when both standards were used for sample detection, we also found deviations between results obtained using each standard serum [5]. Based on these findings, we decided to carry out a series of parallel RFFIT detections using the National Institute of Infectious Disease in Japan (NIID) STD (the standard serum used in the NIID, with a titer of 2 IU/mL) and the WHO STD on four sera samples in collaboration with the NIID laboratory. In this work we present the comparison and analysis results from this cooperation.
HTML
-
CVS-11 is the standard challenge strain of rabies virus used in RFFIT, and is preserved by the laboratory of NIID in Japan; rat neuroma cells (MNA cell) used in RFFIT was also preserved in the NIID laboratory.
-
TI-STD was used as the first standard serum which is preserved by the NIID of Japan, it is usually used to detect anti-rabies neutralizing antibodies at a titer of around 2 IU/mL. The WHO STD was used as the second standard serum (at a titer of 30 IU/mL) and was purchased from the National Institute for Biological Standards and Control (NIBSC) in the UK.
-
MEME medium (Sigma); trypsin, penicillin-streptomycin and fetal bovine serum (FBS) were purchased from Invitrogen; FITC anti-rabies monoclonal globulin was purchased from the FujiRebio Diagnostic Company (Japan).
-
Sample C1 was the NSSC STD, titrated by the National Institute for Food and Drug Control using the CCDC RFFIT with WHO STD as the reference, and an original titer of 21.4 IU/mL [2]. Samples S1, S2 and S4 were tested several times using the NIID RFFIT with TI-STD as the reference, with an original titer of 10.1 IU/mL, 6.13 IU/mL and 2.09 IU/mL respectively.
-
A MNA cell monolayer was digested with trypsin and suspended with MEME-10 medium containing 10% FBS. After counting the density of the cells, 2 mL cell suspension (1×106 cells/mL) was inoculated into a 25cm2 flask, which was cultured in 5% CO2 incubator at 37 ℃.
-
The control system in CCDC RFFIT consisted of: the standard sera (WHO STD and TI-STD), negative serum control, virus control and cell control. The negative serum control consisted of human serum without rabies vaccine inoculation.
The control system in NIID RFFIT consisted of: the standard serum (TI-STD), virus control and cell control.
-
CCDC RFFIT test: the sera samples were denatured at 56℃ for 30 minutes, each serum sample was then diluted as a set of 3-fold series dilutions out to the 12th well of a 96-well plate using MEME-10 medium. TI-STD and CCDC STD, the negative serum control were diluted in the same way. A 2-fold dilution for the virus control was also prepared using MEME-10, with nothing added into the cell control. With the exception of the virus control wells and the cell control wells, CVS-11 suspension (with sufficient quantity to infect 80% of cells in each well) was added into each well. After neutralization in an incubator at 37 ℃ with 5% CO2 for 1 h, 50 µL MNA cell suspension (1×106 cells/mL) was added into each well and the 96-well plate was replaced in the incubator at 37 ℃ with 5% CO2 for 24 h.
NIID RFFIT detection: a 96 well plate was prepared one day in advance at a density of 4-4.5×104 MNA cells in each well. After the cells were incubated in an incubator at 37 ℃ with 5% CO2 for 24 h, the denatured sera samples were subjected to continuous series dilution, (summarized in Table 1); NIID reference serum was diluted in the same way as described above; the virus control was subjected to 10-fold series dilution to the 4th well and each dilution of the virus control was duplicated across four wells. 120 µL of 320-1500 FFD50/mL of CVS-11 virus suspension was added into each well except for the wells of virus control and cell control, and the samples were subjected to neutralization at 35℃ in an incubator with 5% CO2for 90 min.

Table 1. Distribution of the detected serums and controls in NIID RFFIT system in 96 well plate
-
The foci of infectious (fluorescent foci) from CVS-11 was checked with a FITC anti-rabies monoclonal globulin, the detailed procedure was as follows:
CCDC RFFIT detection: the 96-well plate was transferred to a P2 safety cabinet, the medium of cell culture was removed and the cells were rinsed with PBS and fixed with 80% cold acetone for 30 min at 4 ℃, then stained with the FITC anti-rabies monoclonal globulin solution. Finally, 60% glycerol-PBS was added and the fluorescent foci were counted under the microscope at 10× magnification; the percentages of the fluorescent foci in the wells before and after 50% of the cells were infected in the samples for detection were then counted. Tests were repeated by two lab operators (P and M) from the CCDC and NIID respectively, and carried out in parallel three times with all four samples (C1, S1, S2 and S4).
NIID RFFIT detection: after the 96-well plate was fixed and stained, it was observed under the microscope at 200× magnification, ten different visual fields were selected from each well and the numbers of visual fields with fluorescent foci were counted. Tests were repeated two times with three samples (C1, S1 and S4) by the NIID RFFIT.
-
The neutralizing antibody titer was calculated by Microsoft Excel software using the Reed & Muench formula to calculate the 50% end-point dilution of standard serum and samples and the finally titer of the sample serum according to the following formulae:
Reed & Muench formula:
Log (50% end-point dilution)=log (starting point dilution) -'difference of logarithms'
Titer of the sample serum=
×titer of STD
Viruses and cells
Standard sera
Reagents
Sera samples for detection
MNA cell culture
Setting the control system
RFFIT test
Quantifying the fluorescent focus infected by CVS-11 challenge virus:
Calculation of the titer of anti-rabies neutralizing antibody
-
No fluorescent focus was found for the cell control in both of RFFIT tests and more than 50% of the cells were infected in first well of the negative control; the virus control wells reached 80%-90% of fluorescent foci in CCDC RFFIT and the virus titer of the virus control were all within the range of 320-1500FFD50/mL in NIID RFFIT (Fig. 1).
-
The CCDC results for the four reference samples C1, S1, S2 and S4 using TI-STD and CCDC STD and the NIID tests on C1, S1 and S4 are summarized in Table 2.

Table 2. Detection results for the samples using NIID RFFIT and CCDC RFFIT
-
The two-sample t-test was used to compare the titers of the samples obtained by NIID RFFIT and CCDC RFFIT (Table 3). The results indicate that the difference between the tests was not significant at P > 0.05.

Table 3. T-test results for titers detected from NIID RFFIT and CCDC RFFIT
-
Similarly, the two-sample t-test was used to compare the titers obtained by operators P and M using CDC RFFIT. The results are shown in Table 4. At p > 0.05 there was no significant difference in the results obtained by the two operators.

Table 4. T-test results for comparison of titers obained by operators P and M
-
Finally, the two-sample t-test was used to compare the detection titers obtained using TI-STD and WHO STD as the reference serum by CCDC RFFIT and the original titers of the four sample. Results are shown in Table 5.

Table 5. T-test results for comparison of the detection results obtained from TI-STD, WHO STD with the original titer of the detected samples
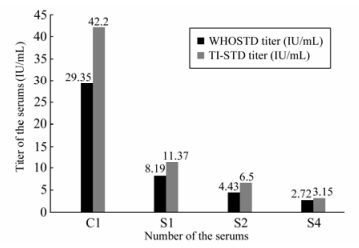
The comparison of the detection result referenced by TI-STD and the original titer of the detected samples using T-test showed a significant difference only for C1 sample; the t-test for the other three samples showed no significant difference. However, comparison of the detection result for WHO STD reference and the original titer of the detected samples showed a significant difference for all the detected samples; a similar result was found when comparing the titers of the two standards (TI-STD, WHO STD) for detection of all four samples.
The Geometric Mean Titers (GMT) of every sample detection result obtained using the two references as the serum standard are summarized in Fig. 2. It can be seen clearly that the GMTs obtained using TI-STD as the reference are higher than those obtained using WHO STD as the reference.
Detection results of controls
Results of sample detection
Comparison and analysis of the detection results between NIID RFFIT and CCDC RFFIT
Comparison and analysis on the results obtained by the two operators
Comparison and analysis of the detection results using TI-STD and WHO STD as the respective reference serum in CCDC RFFIT
-
In 1992 the WHO expert committee for rabies recommended substituting the mouse neutralization test (MNT) for RFFIT for detection of anti-rabies neutralizing antibodies [3]. Thus, as the standard WHO method, the accuracy of the RFFIT is very important.
Our laboratory has already successfully established the RFFIT method and used it widely in China. Our experience to date has been that the stability and reproducibility are very good and previous studies have shown that the results from the same sample tested in different laboratories using the same standard serum had good agreement [4].
However, as consequence of an ongoing cooperation with the NIID, we carried out several tests in parallel on four sera samples to compare the results obtained by two different RFFIT protocols. The results between the two laboratories showed no significant difference, indicating the RFFIT detection had high coincidence between the two laboratories. We also investigated the variation in results obtained by two operators and these also showed no significant difference, indicating results are not affected by operator or reading errors. However, we found significant differences in titers obtained using different standard serums; in particular, the results obtained using TI-STD were significantly higher than those obtained using WHO STD.
C1 is the NSSC STD prepared by the National Institute for Food and Drug Control of China [2], the results obtained using WHO STD and TI-STD as the reference were all higher than the original titer (21.4 IU/mL); a similar conclusion had been obtained in our previous experiments [5].
S1, S2 and S4 are reference samples that are preserved by NIID and which have been tested several times by NIID RFFIT using the TI-STD. The titers obtained using TI-STD was not significantly different from the original titer. However, a significant difference was found when comparing the titers obtained using WHO STD and the original titers; and also between the TI-STD and WHO STD.
One of the WHO recommendations for RFFIT is that the same standard serum should be used in all laboratories, but this is difficult to achieve in practice since purchasing standard serum from WHO is time-consuming and expensive; therefore, many laboratories prepare their own standard serums. Our data indicate that RFFIT results using the same serum sample with different standard serums may be significantly different. Given the number of vaccinations that are carried out and validated by testing, this is a cause for concern and this difference warrants further investigation.














 DownLoad:
DownLoad: