HTML
-
Newcastle disease virus (NDV) and avian influenza virus (AIV) are among the most notorious avian pathogens listed by The World Organisation for Animal Health (OIE) (OIE 2012). The infection of the two viruses causes severe economic losses in the poultry industry worldwide. The AIV strain H9N2 normally causes depression, ruffled feathers, and body weight loss in chickens of all ages, while infection with virulent NDV strains in susceptible animals can lead to mortality as high as 100% (Alexander 2000, 2001; Matrosovich et al. 2001).
NDV belongs to the genus Orthoavulavirus, subfamily Avulavirinae, family Paramyxoviridae, comprising a single negative stranded RNA genome encoding six structural proteins including nucleocapsid protein (NP), phosphoprotein (P), matrix protein (M), fusion protein (F), haemagglutinin–neuraminidase (HN), and the large polymerase protein (L). Among them, the F and HN proteins contribute to major protective antigens (Alexander 2000). Based on the sequence variations of these proteins, NDV strains are sorted into class Ⅰ and class Ⅱ. Class Ⅰ only contains genotype 1, which is lentogenic to both domestic fowls and wild birds, while 21 genotypes (genotype Ⅰ– ⅩⅪ) are found among the Class Ⅱ strains, including the epizootic virulent genotype Ⅶ strains circulating in Asia (Diel et al. 2012; Dimitrov et al. 2019; Liu et al. 2003; Miller et al. 2015; Snoeck et al. 2013; Susta et al. 2014; Xue et al. 2017).
For type A influenza viruses from the family Orthomyxoviridae, the viral genome is a segmented negative RNA strand. The antigenic differences of viral surface glycoproteins, haemagglutinin (HA), and neuraminidase (NA) define the subtypes. Among them, H9N2 is the predominant subtype of AIV in China (Li et al. 2019; Lindh et al. 2014; Liu et al. 2016).
Vaccination is of great importance for the control of viral infection. Vaccines against NDV have been mostly formulated using lentogenic genotype Ⅱ NDV strains (LaSota, B1, and clone 30) obtained decades ago, and whose genetic distances are 18.3%–26.6% to those of current predominant genotype Ⅶ strains (Dimitrov et al. 2016). Although these vaccines are effective in preventing clinical signs, virus shedding from virulent NDV straininfected individuals cannot be fully aborted (Dimitrov et al. 2017; Hu et al. 2009; Miller et al. 2007). For AIV H9N2, the problem of antigenic mismatch of vaccines also exists, and the use of these vaccines exerted continuous mutation pressure on the viral HA gene. The novel genotype 57 (G57) from the clade h9.4.2 lineage (Y280-like) was isolated regularly from vaccinated flocks and was responsible for the H9N2 outbreaks during 2010–2013 (Pu et al. 2015). Therefore, a safe and efficient vaccine candidate is required to control NDV and AIV H9N2.
NDV and AIV normally invade their target avian hosts through the upper respiratory mucosa, where they accomplish their original replication and subsequently spread systemically. Therefore, the fast and powerful responses of the mucosal immune system are crucial to resist these pathogens. Among the mucosal residential immune cells, dendritic cells (DCs) are a specialized family with potent antigen presentation ability that effectively bridges innate and adaptive immunity (Lee and Iwasaki 2007). DCs are recruited to pathogen invasion sites to intake, process, and present the antigens, thus activating naïve T or B cells into effector cells, leading to secretory immunoglobulin A (sIgA) production in mucous membranes (Bemark et al. 2012). SIgA secretion constitutes the main molecular part of the antigen-specific barrier. As our understanding of function of DC in the mucosal immune system expands, DCs are becoming a promising target for vaccines to improve mucosal immune activation. And the DC-binding peptide (DCpep) is a short specific DC-targeting peptide that functions as an immune enhancer by speeding up DC recruitment and the intake of pathogens (Mohamadzadeh et al. 2008, 2009).
Virus-like particles (VLPs) are assemblies of viral structural proteins with no incorporation of the viral genome and are ideal candidate vaccines because of their wellpreserved antigenicity and guaranteed safety. Previously, our team constructed NDV VLPs by packaging NDV structural M and HN proteins using a Bac-to-Bac baculovirus expression system. The NDV VLPs displayed enhanced protection against NDV through the activation of DCs in mice (Jing et al. 2017). In the present study, to generate a suitable bivalent candidate vaccine with convincing dual protection ability against genotype Ⅶ NDV and AIV H9N2 (genotype 57), DCpep-decorated chimeric VLPs (cVLPs) were constructed. The cVLPs were named as H9/F-DCpep-cVLPs, comprising the NDV M protein as the skeleton, the NDV HN protein, and DCpep-fused H9N2 HA protein co-expressed on the particle surfaces. Their ability to induce chicken bone marrow-derived dendritic cell (chBMDC) maturation was tested in in vitro. Their antigenicity to generate NDV- and AIV H9N2-specific HI antibodies and sIgA to induce active T cell proliferation, and their protection against NDV NA-1 strain (genotype Ⅶ), Herts/33 strain (genotype Ⅳ), and AIV H9N2 (genotype 57) were determined in SPF chickens.
-
NDV NA-1 strain (GenBank: DQ659677) was isolated and characterized in our laboratory (Xu et al. 2008). The international standard NDV Herts/33 strain (GenBank: AY741404) was obtained from the China Institute of Veterinary Drug Control (Beijing, China). The AIV H9N2 A/chicken/Jilin/SJ150/2012 strain (GenBank: KF886457) was kindly provided by Prof. Yanlong Cong (Jilin University, Jilin, China). NDV and AIV were propagated in the allantoic cavities of 9-day-old embryonated chicken eggs, collected after 72 h, and purified using density gradient centrifugation. Briefly, NDV and AIV were condensed through polyethyleneglycol (PEG) precipitation and centrifuged in a 60%–40%–20% sucrose gradient at 50, 000 ×g for 1 h and washed with PBS. The virus was titrated using a standard haemagglutination test (HA test), and the embryonic 50% lethal dose (ELD50) was calculated as described previously (Luginbuhl and Jungherr 1949). Recombinant baculoviruses expressing the NDV M and HN genes (rBV-M and rBV-HN) were constructed in our laboratory (Jing et al. 2017).
The Bac-to-Bac insect-baculovirus expression system (Life Technologies Corporation, Gaithersburg, MD, USA) consisting of the pFastBac1 transfer vector, Escherichia coli DH10Bac competent cells, and insect Sf9 cells, was stored in our laboratory. chBMDCs were collected from 10 day-old specific pathogen-free (SPF) chicken femurs and tibias, and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% foetal bovine serum (FBS, Gibco, Gaithersburg, MD, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, 20 ng/mL chicken granulocyte–macrophage colony-stimulating factor (GM-CSF), and 20 ng/mL chicken interleukin-4 (IL-4) (Kingfisher Biotech, Saint Paul, MN, USA) for 7 days (Liang et al. 2013). The chBMDCs were stimulated with lipopolysaccharide (LPS) (10 μmol/L) for 24 h, and subsequently measured by mean fluorescence intensity (MFI) for the maturation of the surface markers CD11c and major histocompatibility class-Ⅱ (MHC-Ⅱ) protein using flow cytometry.
SPF chicken eggs and SPF chickens (White Leghorn) were purchased from Beijing Merial Vital Laboratory Animal Technology Co., Ltd (Approval number SCXKJing-2015-0002) and hatched under routine laboratory conditions with free access to feed.
-
NDV F (derived from the NA-1 strain) and AIV HA (derived from the H9N2 A/chicken/Jilin/SJ150/2012 strain) sequences were determined by transmembrane region analysis software (TMHMM Server v. 2.0). A fusion sequence (H9/F) encoding the HA ectodomain and F transmembrane domain was constructed using overlap polymerase chain reaction (PCR). The DCpep sequence (FYPSYHSTPQRP) and melittin signal peptide (from Baculovirus expression vector pFastBac1-HM, GenBank: AY598466) were cloned to the 5′ terminal of H9/F, and subsequently inserted into the pFastBac1 vector via the Sal Ⅰ and Kpn Ⅰ sites. Finally, the rBV-H9/F and rBV-H9/F-DCpep were generated using the Bac-to-Bac insect-baculovirus expression system.
-
The H9/F-cVLPs were produced by co-infecting of rBV-M, rBV-HN, and rBV-H9/F at a multiplicity of infection (MOI) ratio of 5:4:4 at 27 ℃ for 72 h. H9/F-DCpep-cVLPs were produced by the co-infection rBV-M, rBV-HN, and rBV-H9/F-DCpep. The infected supernatants were harvested and purified using sucrose gradient ultracentrifugation (OIE 2012). The purified cVLPs were quantified using a Pierce BCA Protein Assay Kit (Pierce Thermo Scientific, Rockford, IL, USA). The morphology and structure of the purified cVLPs were observed on a Hitachi H-7650 transmission electron microscope (TEM) after negative staining (Hitachi Ltd, Tokyo, Japan) operating at 80 kV. For Western blotting analysis, protein bands on polyacrylamide gels were transferred to polyvinylidene difluoride (PVDF) membranes and then incubated with a 1:500 dilution of chicken anti-NDV polyclonal sera or chicken anti-H9 polyclonal sera (Weike Biotechnology Co. Ltd, Harbin, China) as primary antibodies, followed by a 1:5000 dilution of horseradish peroxidase (HRP)-conjugated goat antichicken IgY (EarthOx Biotechnology, San Francisco, CA, USA) as the secondary antibody. Visualization of the immunoreactive proteins was conducted using enhanced chemiluminescence (ECL) with an ECL Plus detection kit (Thermo Fisher Scientific, Waltham, MA, USA).
-
The chBMDCs were plated at 5 × 106 cells/well into 6-well plates and treated separately with phosphate-buffered saline (PBS), LPS (10 μmol/L), H9/F-cVLPs (10 μg), and H9/F-DCpep-cVLPs (10 μg) for 24 h. These BMDCs were further supplemented with 1 mg/mL fluorescein isothiocyanate (FITC)-dextran (40 kDa, SigmaAldrich, St. Louis, MO, USA) at 37 ℃ for 2 h and washed with PBS (pH 7.2) twice, before being subjected to flow cytometry to measure MFI. The MFI of chBMDCs kept at 4 ℃ served as the negative control. The uptake ability was described by ΔMFI = MFI (37 ℃) - MFI (4 ℃) (Jing et al. 2017). Apart from the ΔMFI, another set of chBMDCs was treated with H9/F-cVLPs, H9/F-DCpep-cVLPs, LPS, or PBS for 24 h at 37 ℃, incubated with phycoerythrin (PE)-conjugated anti-chicken MHC-Ⅱ antibodies (Abcam, Cambridge, UK), and subjected to flow cytometry.
-
SPF chickens (n = 231; 7 days old) were housed in an animal biosafety level 2 facility (ABSL2, Jilin University). They were randomly distributed into seven groups (n = 33). The bivalent commercial vaccine (inactivated NDV LaSota strain and A/Chicken/Shanghai/F/98 (H9N2)) was purchased from Qian Yuanhao Biological Co., Ltd. (Beijing, China; Veterinary drug approval number, 2014-100082179). The vaccination dose of cVLPs was 40 μg in 100 μL of PBS per chicken. The commercial vaccine was administered intramuscularly following the supplier's protocol.14 days after boosting, 27 chickens from each group were further divided into three groups (n = 9) and intranasally challenged with NDV NA-1 strain (1 × 102 ELD50), Herts/33 strain (1 × 102 ELD50), or AIV H9N2 (1 × 105 EID50) in 100 μL of PBS, respectively, with six chickens from each immunization group remaining unchallenged for collection of bronchoalveolar lavage fluid (BALF) and splenic cells. The scheme for the vaccination and challenge of chickens was designed as described in Table 1.
Group Antigen Doses (per) Primary immunisationa Boost immunisation Immunisation route Challengeb PBS-IN PBS 200 μL Day 7 Day 21 Intranasal immunisation Day 35 PBS-IM PBS 200 μL Day 7 Day 21 Intramuscular injection Day 35 cVLPs-IN H9/F-cVLPs 40 μg in 200 μL Day 7 Day 21 Intranasal immunisation Day 35 cVLPs-IM H9/F-cVLPs 40 μg in 200 μL Day 7 Day 21 Intramuscular injection Day 35 DCpep-cVLPs-IN H9/F-DCpep-cVLPs 40 μg in 200 μL Day 7 Day 21 Intranasal immunisation Day 35 DCpep-cVLPs-IM H9/F-DCpep-cVLPs 40 μg in 200 μL Day 7 Day 21 Intramuscular injection Day 35 Commercial vaccine Commercial vaccine 200 μL Day 7 Day 21 Intramuscular injection Day 35 aSeven-day-old SPF chickens were immunised with different antigens.
bOn Day 35 (14 days after boosting), immunised groups were challenged with the virulent NDV NA-1 strain, the NDV Herts/33 strain, or the AIV H9N2 A/chicken/Jilin/SJ150/2012 strain via the intranasal or intramuscular route.Table 1. Program of vaccination and challenge.
-
After primary immunisation, serum samples were collected every 2 days and tested with haemagglutination inhibition (HI) to monitor NDV and AIV-specific antibody changes. The HI assay was performed using 1% chicken red blood cells (RBC) with four haemagglutination units (HAU) of standard NDV LaSota and H9 AIV antigens (Weike Biotechnology Co. Ltd, Harbin, China) following the standard method (OIE 2012). Two weeks after boosting (Day 35), six chickens from each group were euthanized to collect BALF and splenic cells. BALF was then subjected to an enzyme-linked immunosorbent assay (ELISA) to evaluate AIV and NDV-specific secretory IgA levels. Briefly, 500 ng/well of inactivated NDV or AIV (generated from live virus through UV exposure for 30 min) was applied to 96-well-microplates (Costar, Corning, NY, USA) with coating buffer (0.05 mol/L sodium carbonate, pH 9.5) at 37 ℃ for 2 h. After washing with PBS containing 0.5% Tween-20 (PBST), the plates were blocked with PBS containing 1% bovine serum albumin at 37 ℃ for 1 h and incubated with a 1:200 dilution of each sample at 37 ℃ for 1 h. After washing with PBST five times, all samples were incubated for 1 h with a 1:5000 dilution of HRP-conjugated goat anti-chicken IgA (EarthOx Biotechnology, Millbrae, US) as the secondary antibodies. Then, 100 μL of the 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate solution (Tiangen Biotech Co. Ltd., Beijing, China) was added and the samples were incubated at 37 ℃ for 15 min in the dark. The enzyme reaction was stopped after 15 min by the addition of 50 μL stop solution (2 mol/L H2SO4), and the absorbance was measured at 450 nm. IgA was calculated using the following formula: Y = 12.138X - 1.7731, where Y is the concentration of IgA and X is OD450nm value.
-
The splenic T lymphocyte differentiation was performed as previously described with slight modifications (Jing et al. 2017). Briefly, the spleens were aseptically isolated, ground, filtered, treated with lymphocyte separation medium (Solarbio, Beijing, China), rinsed with chicken RBC lysis buffer, and suspended in RPMI-1640 to adjust the cell density to 1 × 106 cell/mL. Lymphocytes were incubated with fluorescent antibodies and isotype controls (Abcam, Cambridge, UK): PE anti-chicken CD3, FITC anti-chicken CD4, and Allophycocyanin (APC) anti-chicken CD8a antibodies. Flow cytometry was used to measure the percentage of cells positive for surface markers in the samples.
-
After the viral challenge, the survival rate and body weight (BW) changes were monitored for 14 days. Chickens were euthanized, and samples from the brain, lung, heart, pancreas, spleen, glandular stomach, breast, and thigh muscle (approximately 0.125 cm3) were taken at 3, 5, and 7 days post-challenge (dpc). The samples were homogenized, and the supernatants were inoculated into 9-day-old embryonated chicken eggs to detect tissue virus titres. Oropharyngeal and cloacal swabs were also collected to monitor virus shedding. At 72 h after inoculation into embryonated chicken eggs, the allantoic fluid was collected, and the virus was tittered using a standard HA assay. The samples with HA values higher than 4log2 were recorded as positive.
-
The experimental data between two groups were analysed using one-way analysis of variance (ANOVA) in SPSS (version 13.0), and graphs were constructed using GraphPad Prism (Version 6) software. In all analyses, a P value of less than 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001) and a P-value greater than 0.05 indicated no significant difference (ns). All results are representative of at least three repeated experiments. In the figures, error bars represent the standard deviation (SD).
Viruses, Cells, and Animals
Construction of Recombinant Baculovirus
Production and Identification of Chimeric VLPs
Detection of DC Activation in vitro
Vaccination and Challenge
Antibody Response Assessment
Analysis of T Lymphocyte Differentiation
Evaluation of Protective Efficacy
Statistical Analysis
-
The rBVs were constructed to express chimeric HA protein H9/F or H9/F-DCpep (Fig. 1A). The insect baculovirus expression system was employed to produce chimeric NDV VLPs (cVLPs) via the co-infection of rBV-M, rBVHN with rBV-H9/F or rBV-H9/F-DCpep. TEM identification showed that both H9/F-cVLPs and H9/F-DCpep-cVLPs had spherical shapes and were covered by an intact membrane and coated with spikes, with sizes ranging from 100 to 200 nm in diameter (Fig. 1B, 1C). Furthermore, Western blotting detected the expression of NDV M (40 kDa) and HN (68 kDa) in both H9/F-cVLPs and H9/F-DCpep-cVLPs using anti-NDV chicken polyclonal antibody, as predicted (Fig. 1D, 1E). Meanwhile, chimeric H9/F (65 kDa) and H9/F-DCpep (65 kDa) were also detected in H9/F-cVLPs or H9/F-DCpep-cVLPs using an anti-H9 serum (Fig. 1F, 1G).
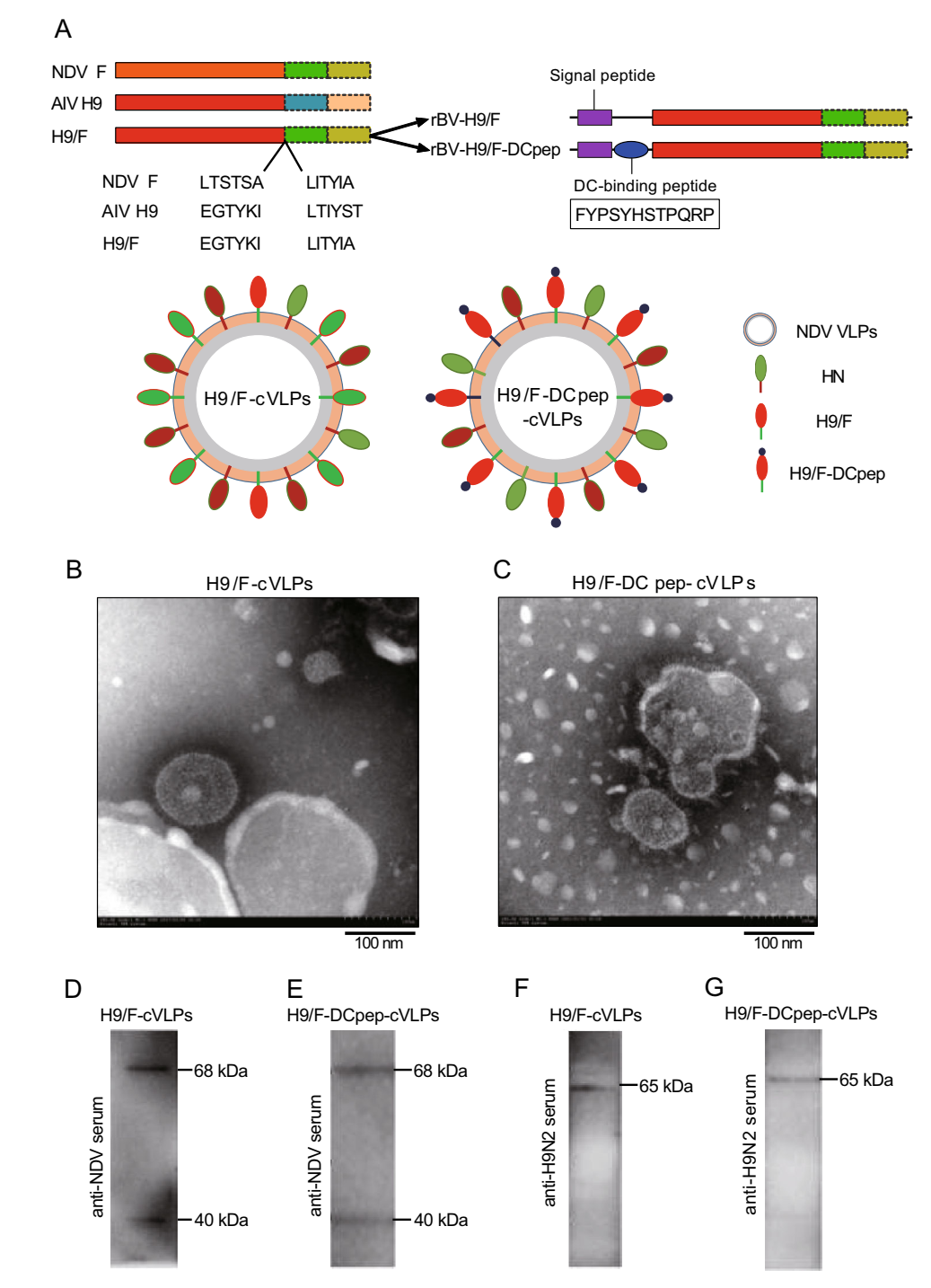
Figure 1. Construction diagram of recombinant baculovirus (rBV) and identification of cVLPs. A The ectodomain of NDV F protein was replaced with that of AIV H9 protein (H9/F). DCpep sequence was fused to the amino terminal of H9/F protein through a GS-linker and co-expressed with NDV HN protein on the surface of NDV VLPs. B, C Negative staining TEM of H9/F-cVLPs and DCpep-H9/F-cVLPs. D, E, F, G Western blot analysis of cVLPs through mouse anti-NDV and anti-H9N2 polyclonal serum. The molecular weight (MW) of M, H9/F, DCpep-H9/F and HN were approximately 40, 65, 65 and 68 kDa, respectively.
-
After culturing with supplemental IL-4 and GM-CSF, chBMDCs were successfully derived from the chicken femurs and showed an irregular round shape with short tentacles protruding on their surfaces (Fig. 2A). These immature chBMDCs expressed low levels MHC-Ⅱ on their surfaces (Fig. 2B). After stimulation with LPS for 48 h, these cells exhibited a stellate-like characteristic with long and thin dendritic tentacles (Fig. 2A), together with increased MHC-Ⅱ levels (Fig. 2B), suggesting that LPS could activate the maturation of chBMDCs. The expression of CD11c also displayed slightly increase after LPS treatment.
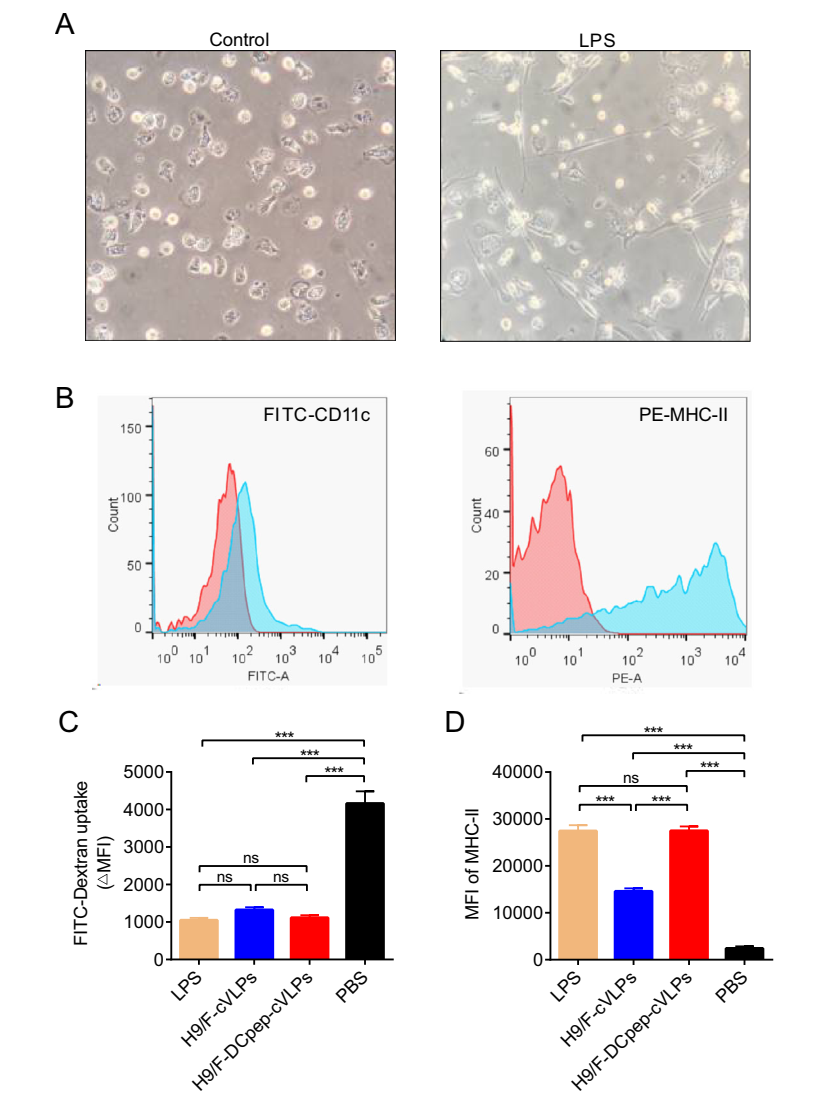
Figure 2. The cVLPs induced chBMDC maturation. A Observation of naïve (PBS control) and mature (LPS) chBMDC. The naïve DCs exhibited an irregular round shape with short tentacles and mature DCs exhibited a typical stellate-like shape with long dendritic tentacles. B Maturation markers (CD11c and MHC-Ⅱ) on the surface of naïve (red) and mature (blue) chBMDCs, indicated by cell counts of fluorescence positive population. C The ΔMFI value of FITC-Dextran treated chBMDCs incubated with LPS, H9/F-cVLPs, H9/F-DCpep-cVLPs and PBS were detected through flow cytometry and calculated. D The MHC-Ⅱ level of differently stimulated chBMDCs was analyzed through flow cytometry. P value less than 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001) and a P value greater than 0.05 means no significant difference (ns). All results are representative of at least five repeated experiments. Error bars indicate standard deviation (SD).
To test their ability to activate chBMDCs, the cVLPs were incubated with naïve chBMDCs. Another index for DC maturation is their antigen uptake ability. After treatment with a potent DC maturation activator, FITC-dextran intake decreases, which is demonstrated by a decreased ΔMFI value. The H9/F-DCpep-cVLPs-treated BMDCs (ΔMFI = 1114.34 ± 67.35) exhibited a slightly lower uptake ability than the H9/F-cVLPs group (ΔMFI = 1322.33 ± 71.46). The PBS group showed a significantly greater intake ability (ΔMFI = 4160.00 ± 326.50) than the three experimental groups (Fig. 2C). In terms of the DC maturation marker MHC-Ⅱ, all the experimental groups exhibited significantly elevated MHC-Ⅱ levels (Fig. 2D), while the MHC-Ⅱ level of the H9/F-cVLPs group was lower than that of the H9/F-DCpep-cVLPs and LPS groups. Therefore, both H9/F-cVLPs and H9/F-DCpep-cVLPs efficiently activated chBMDCs. H9/F-DCpep-cVLPs more effectively reduced the DC uptake and increased MHC-Ⅱ expression than H9/F-cVLPs, which is likely a result of the DCpep modification.
-
The function of the DCpep modification was confirmed. Therefore, we further tested the immune stimulating ability of H9/F-cVLPs and H9/F-DCpep-cVLPs in chickens. NDV and AIV-specific antibodies were measured through HI tests. Although lacking the assistance of an adjuvant, H9/F-cVLPs and H9/F-DCpep-cVLPs groups showed comparable serum NDV and AIV H9N2 specific antibody levels to those of the commercial vaccine (Fig. 3). After challenge by the NDV NA-1, Herts/33, and AIV H9N2, the HI value of the H9/F-cVLPs and H9/F-DCpep-cVLPs groups decreased at first, which was attributed to the matched genotype of the virus strain, and then the antibody level recovered soon after (Fig. 3). In line with the potent DC activating ability of DCpep, the H9/F-DCpep-cVLPs group demonstrated higher levels of antibodies against AIV H9N2, NDV NA-1 strain, and Herts/33 strain (Fig. 3). Notably, the immunisation route also significantly influenced the immune response: the two cVLPs-IM groups generated higher HI values than the VLPs-IN group.
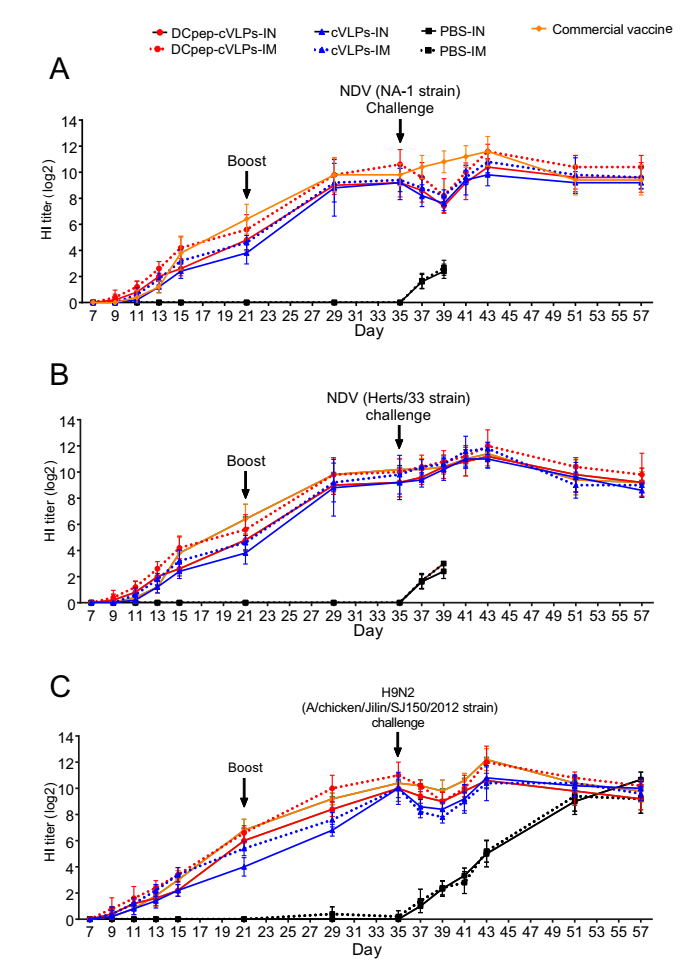
Figure 3. Serum NDV and AIV H9N2 specific HI antibody change after immunization in chickens. H9/F-cVLPs, DCpep-H9/F-cVLPs and PBS were intranasally (IN) or intramuscularly (IM) administered at Day 7, boosted at Day 21 and challenged with respective virus strains at Day 35. Commercial bivalent vaccine was administered intramuscularly. The serum NDV NA-1 strain (A), Herts/33 strain (B) and AIV H9N2 (C) specific antibody was monitored for 57 days.
AIV and NDV mainly attack their host through the upper respiratory system; therefore, the mucosal sIgA level is pivotal as the first barrier against pathogens. We tested the mucosal sIgA level in BALF and found that all the experimental groups demonstrated profound sIgA secretion compared with that in the PBS group, and that of cVLPs groups surpassed the commercial vaccine group (Fig. 4A, 4B). Although intranasally administered (IN) DCpep-H9/F-cVLPs showed no obvious advantages over the intramuscular (IM) group in terms of serum antibody levels (Fig. 3), the sIgA levels in the BALF were significantly higher in the IN groups compared with those in the IM immunised group (Fig. 4A, 4B). Therefore, both H9/F-cVLPs and H9/F-DCpep-cVLPs stimulated sufficient protective antibodies against NDV Herts/33, NDV NA-1, and AIV H9N2, and the intranasal immunisation route enhanced BALF sIgA secretion.
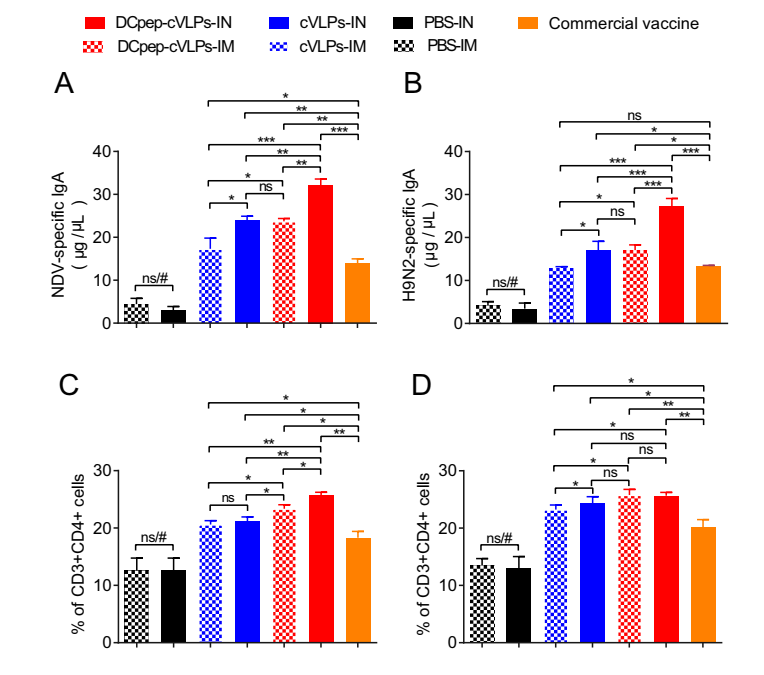
Figure 4. The cVLPs induced bronchoalveolar lavage fluid (BALF) sIgA secretion and splenic T cell proliferation. BALF was collected 2 weeks after boost and examined for NDV (A) and H9N2 (B) specific sIgA antibody level through ELISA. CD3 + CD4 + cells (C) and CD3 + CD8 + cells (D) cell percentage in spleen was determined through flow cytometry. P value less than 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001) and a P value greater than 0.05 means no significant difference (ns). All results are representative of at least six repeated experiments. Error bars indicate standard deviation (SD). "#" represents significant differences between PBS group and other groups.
In addition to antibody secretion, the cellular immune response is also critical in the process of virus elimination. We evaluated T cell immune responses of CD3 + CD4 + cell (Th) and CD3 + CD8 + cell (Tc) subsets in the spleen. The results showed that the splenic Th percentage was significantly higher in the H9/F-DCpep-cVLPs-IM group (23.37% ± 0.41%) than in the H9/F-cVLPs-IM group (20.6% ± 0.40%), both of which were better than that of the commercial vaccine group (18.3% ± 1.25%) (Fig. 4C). The immunisation route did not alter the Th population of the H9/F-cVLPs groups, while the Th level of the H9/F-DCpep-cVLPs-IN group was significantly higher than that of the H9/F-DCpep-cVLPs-IM group (Fig. 4C). The Tc population showed the same tendency as the Th population. The Tc percentages of the H9/F-DCpep-cVLPs-IM, H9/F-cVLPs-IM, commercial vaccine, and PBS groups were 25.8% ± 0.55%, 23.23% ± 0.46%, 20.13% ± 0.78%, and 14.1% ± 0.60%, respectively (Fig. 4D). The IN route slightly increased Tc proliferation in the H9/F-cVLPs group, but not in that of the H9/F-DCpep-cVLPs group (Fig. 4D). Therefore, DCpep modification could increase Tc and Th proliferation to a limited extent, and their population in the IN groups were comparable or slightly higher than those in the IM groups.
-
The survival rate, body weight change and virus shedding of tested SPF chickens were monitored for 14 days after virus challenge (Fig. 5). All chickens in the NDV-infected PBS group died in 5 days, while 80% of the H9N2-infected PBS group survived to the end of the experiment. For the experimental groups, cVLPs and the commercial vaccine provide 100% protection (Fig. 5A, 5D, 5G). However, the BW of groups vaccinated with different VLPs varied. At the end of the experiment, the BW in the cVLPs groups increased by 20% when challenged with the virulent NA-1 strain, while that of commercial vaccine group increased by only 13.6% (Fig. 5B). When infected with a virulent Herts/33 strain, the BW of the cVLPs-IN group increased by 15.8%, which was notably higher than of the IM cVLPs (11.1%) (Fig. 5E). After AIV H9N2 challenge, the BW of the H9/F-DCpep-cVLPs group increased by 18.1%, while that of the surviving subjects in the PBS group remained unchanged (Fig. 5H).
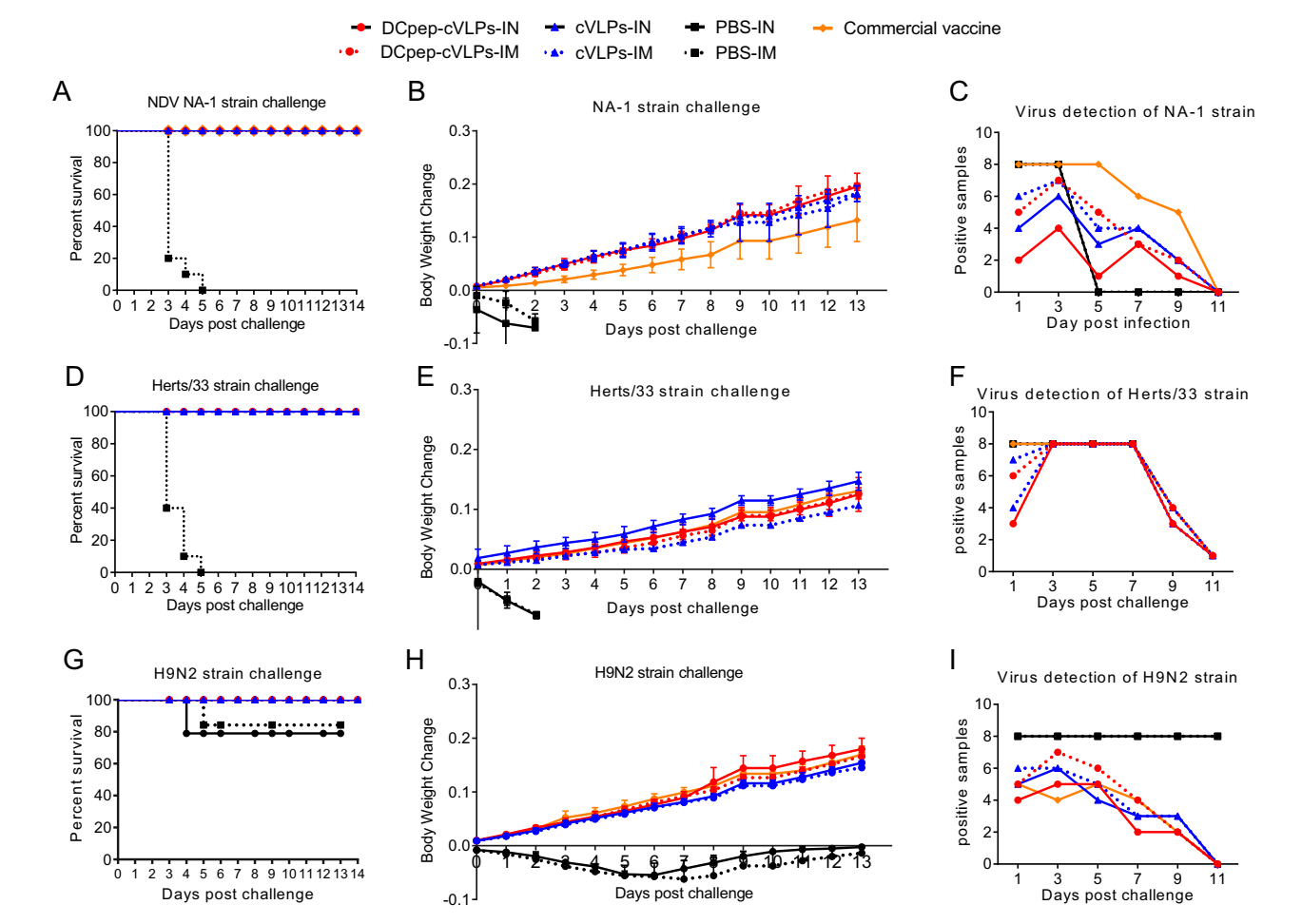
Figure 5. Protective efficacy of cVLPs against NDV NA-1 strain, Herts/33 strain and AIV H9N2 challenge. Survival rate (A), Body weight change (B) and the number of positive tissue samples (C) were recorded after NDV NA-1 strain challenge. Survival rate (D), Body weight change (E) and the number of positive tissue samples (F) were recorded after NDV Herts/33 strain challenge. Survival rate (G), body weight change (H) and the number of positive tissue samples (I) were recorded after AIV H9N2 challenge.
For tissue virus detection and virus shedding, nine tissue samples, including lung, brain, heart, spleen, small intestine, glandular stomach, muscle, and pancreas were collected. Meanwhile, virus shedding was monitored through viral detection in oropharyngeal and cloacal swabs. The numbers of positive samples were recorded. The results showed that, when challenged with NDV or AIV H9N2, fewer positive tissue samples were identified from the H9/F-DCpep-cVLPs-IN group than from the other groups, while immunisation with the commercial vaccine resulted in the highest number of positive samples (Fig. 5C, 5F, 5I). The NDV Herts/33 strain infection contributed to a 100% positive rate in all groups from day 3, which decreased to 11% at day 11, and showing no obvious differences among the experimental groups (Fig. 5F).
In terms of virus shedding, H9/F-DCpep-cVLPs-IN had the shortest virus-shedding period in both oropharyngeal and cloacal samples. Oropharyngeal swabs were only positive for the NDV Herts/33 strain 5 days post-challenge, and the rest of the samples were all negative for both NDV and AIV at all time points (Tables 2, 3, and 4). The commercial vaccine group showed the longest virus-shedding period; oropharyngeal swab samples were positive for the NDV Herts/33 strain until Day 9 (Table 3). H9/F-DCpep-cVLPs-IM treatment resulted in the second shortest virus shedding, and the values for the H9/F-cVLPs groups were between those of the H9/F-DCpep-cVLPs and commercial vaccine groups.
Groupa Oropharyngeal swabs Cloacal swabs 5 dpc 7 dpc 9 dpc 5 dpc 7 dpc 9 dpc PBS-IN 6/6 – – 6/6 – – PBS-IM 6/6 – – 6/6 – – cVLPs-IN 1/9 0/9 0/9 0/9 0/9 0/9 cVLPs-IM 1/9 0/9 0/9 0/9 0/9 0/9 DCpep-cVLPs-IN 0/9 0/9 0/9 0/9 0/9 0/9 DCpep-cVLPs-IM 1/9 0/9 0/9 0/9 0/9 0/9 Commercial vaccine 5/9 3/9 0/9 4/9 2/9 0/9 aThe immunised chickens were challenged with the virulent NDV NA-1 strain. "dpc" represents days post-challenge.
"–" indicates chickens that died in the PBS-treated groups.Table 2. Detection of virus shedding from oropharyngeal and cloacal swabs after NDV NA-1 strain challenge.
Groupa Oropharyngeal swabs Cloacal swabs 5 dpc 7 dpc 9 dpc 5 dpc 7 dpc 9 dpc PBS-IN 6/6 – – 6/6 – – PBS-IM 6/6 – – 6/6 – – cVLPs-IN 4/9 2/9 0/9 1/9 0/9 0/9 cVLPs-IM 4/9 2/9 0/9 5/9 1/9 0/9 DCpep-cVLPs-IN 2/9 0/9 0/9 0/9 0/9 0/9 DCpep-cVLPs-IM 1/9 1/9 0/9 0/9 0/9 0/9 Commercial vaccine 7/9 4/9 1/9 3/9 2/9 0/9 aThe immunised chickens were challenged with the virulent NDV Herts/33 strain. Dpc represents days post-challenge.
"–" represents chickens that died in the PBS groups.Table 3. Detection of virus shedding from oropharyngeal and cloacal swabs after NDV Herts/33 strain challenge.
Groupa Oropharyngeal swabs Cloacal swabs 5 dpc 7 dpc 9 dpc 5 dpc 7 dpc 9 dpc PBS-IN 6/6 6/6 6/6 6/6 6/6 6/6 PBS-IM 7/7 7/7 7/7 7/7 7/7 7/7 cVLPs-IN 1/9 0/9 0/9 0/9 0/9 0/9 cVLPs-IM 2/9 1/9 0/9 0/9 0/9 0/9 DCpep-cVLPs-IN 0/9 0/9 0/9 0/9 0/9 0/9 DCpep-cVLPs-IM 1/9 0/9 0/9 0/9 0/9 0/9 Commercial vaccine 2/9 1/9 0/9 1/9 0/9 0/9 aThe immunised chickens were challenged with the AIV H9N2 A/chicken/Jilin/SJ150/2012 strain. "dpc" represents days postchallenge. Table 4. Detection of virus shedding from oropharyngeal and cloacal swabs after AIV H9N2 challenge.
Characterization of H9/F-cVLPs and H9/F-DCpep-cVLPs
Demonstration of cVLPs Activation of chBMDC in vitro
cVLPs Induced Profound Immune Responses in vivo
cVLPs Produced Full Protection Against Both Homologous and Heterologous Strains
-
Newcastle disease is one of the most devastating avian diseases, and its control mostly depends on prophylactic immunisation regimes. The prevalent NDV strain in China comes from genotype Ⅶ and can be neutralized by current genotype Ⅱ vaccines, which prevent clinical signs. However, the virus load and shedding, owing to the unmatched genotype vaccination regime, pose a great danger for the spreading of pathogens (Miller et al. 2007). In our previous study, a safe, effective, and genotype-matched novel NDV vaccine was constructed, comprising non-productive NDV VLPs derived from structural viral proteins (Jing et al. 2017). In our study, the efficiency of NDV VLPs was demonstrated in SPF chickens, and their potential as a vector platform was further investigated regarding the control of H9N2 AIV. The NDV-based chimeric H9N2 VLPs generated in this study induced a comparable serum AIV H9N2-specific HI titre (24 to 210) to that of the AIV-based H9N2 VLP (24 to 28) (7-days post boost), which was better than that achieved by the commercial H9N2 vaccine (Li et al. 2017). Several bivalent vaccines against the prevalent strains of NDV and AIV H9N2 formulated with the inactivated virus have been reported, all of which exert a full immune activation ability under the assistance of adjuvants (Naggar et al. 2017; Zhao et al. 2017). Although both bivalent cVLPs-based and inactivated virus-based vaccines can completely protect animals from H9N2 shedding and stimulate a sufficient antibody response, there is a slight difference in terms of NDV shedding. When infected with a homologous NDV strain, the protection rate (survival without virus shedding) of H9/F-DCpep-cVLPs-IN reached 100% in our study, while that of inactivated NDV was around 90% (Naggar et al. 2017; Zhao et al. 2017). Of note, the commercial vaccine is supplemented with the adjuvant, which greatly improves the efficacy, while cVLP is administered without the adjuvant. Therefore, even without adjuvant enhancement, cVLPs functioned equivalently to inactivated vaccines in terms of antibody response and virus shedding.
In addition to the package of antigens, our cVLPs were inserted with an immune enhancer, DCpep. Although DCpep would not alter the subtypes or the functions of DCs, it could facilitate DC recruitment to the pathogen invasion sites (Curiel et al. 2004). Antigens modified with DCpep at their amino termini resulted in high avidity of murine CD3 + CD4 + T cell proliferation and homing to sites of antigen deposition in vitro and in vivo (Erskine et al. 2011). Taking advantage of its ability to activate DC, the application of DCpep has been used widely to decorate candidate vaccines against digestive or nervous system pathogens (Hou et al. 2018; Jiang et al. 2015; Wang et al. 2017; Yang et al. 2017; Zhang et al. 2017). In these studies, the DCpep-fused antigen promoted intestinal adhesion, stimulated more TFH, and offered improved systemic IgG and intestinal mucosal sIgA secretion. The sIgA level in the respiratory system is as crucial as its counterpart in the digestive system for pathogen elimination. Unlike the DC-independent secretion of systemic IgA, the production of respiratory IgA requires the participation of DCs (Bessa et al. 2009). To confirm the proficiency of the DCpep modification in our study, chBMDCs were stimulated with H9/F-cVLPs and H9/F-DCpep-cVLPs, which resulted in significantly different antigen-uptake levels and MHCII expression (Fig. 2C, 2D). The DCpep modification remarkably reduced the antigen uptake of chBMDCs and increased their MHC-Ⅱ expression (Fig. 2C, 2D), highlighting the competence of DCpep to activate DCs, which agreed with the results of a previous study (Curiel et al. 2003). In addition to their in vitro efficacy, H9/F-DCpep-cVLPs also showed advantages over H9/F-cVLPs in the in vivo assessment. H9/F-DCpep-cVLPs encouraged stronger cellular immunity than did H9/F-cVLPs, as indicated by the higher percentages of splenic Th and Tc cells (Fig. 4C, 4D). The brisk immune cell activity ultimately contributed to satisfactory antibody production, with the H9/F-DCpep-cVLPs group showing continuously higher serum HI levels than the H9/F-cVLPs group. In addition, intranasal administration further enhanced these differences (Fig. 3). The determined sIgA secretion level in BALF also demonstrated the benefit of DC activation. These results showed that the DCpep modification stably increased H9N2 and NDV-specific sIgA secretion, regardless of the route of immunisation (Fig. 4A, 4B), which constructed a solid barrier against respiratory attack. The results of the challenge test further supported the rationale of applying DCpep. When birds were immunised through the same route, H9/F-DCpep-cVLPs produced an overall shorter period of oropharyngeal and cloacal virus shedding, even when challenged with the heterologous Herts/33 strain (Table 3). In general, the DCpep modification of VLPs is effective for improving immune responses.
A convenient immunisation method is crucial for the wide application of vaccines in large-scale poultry production. In our study, the effectiveness of intranasal immunisation was also tested. In accordance with their well-confirmed intestinal mucosal immune-activating ability (Hou et al. 2018; Wang et al. 2017; Yang et al. 2017), both intranasal H9/F-DCpep-cVLPs and H9/F-cVLPs immunisation offered notably increased IgA secretion in the respiratory system compared with that induced by intramuscular (IM) administration (Fig. 4A, 4B). Although the HI titre of the IN groups was slightly lower than that of the IM groups, it remained above the protection level to the end of the experiment (Fig. 3). Furthermore, the DCpep-cVLPs-IN group had the shortest virus shedding period when infected by the NDV NA-1 and AIV H9N2 strains: virus shedding was fully aborted from 5 days post-challenge (Tables 2, 4).
In this study, we successfully produced DCpep-modified chimeric genotype-matched NDV VLPs containing the HA antigen of AIV H9N2. In vitro stimulation tests demonstrated the chBMDC-activating efficacy of both H9/F-DCpep-cVLPs and H9/F-cVLPs. Immunisation tests in SPF chickens further confirmed the benefit of DCpep modification via robust sIgA secretion, an enhanced cellular immune response, and elevated protective serum IgG production. Notably, we found that intranasal immunisation induced a shorter virus-shedding period and no changes in body weights compared with those induced by intramuscular administration. In general, the cVLPs could offer full protection against the predominant circulating strains. Meanwhile, DCpep modification significantly improved the mucosal immune stimulation of cVLPs, and intranasal administration could further improve their mucosal immunogenicity, offering a promising candidate vaccine to control and eliminate NDV and AIV H9N2.
-
This work was supported by grants from the National Key Research and Development Program of China (Grant No. 2018YFD0500100), the National Natural Science Foundation of China (Grant Nos. 31772735, 31472195), the Jiangsu Provincial Natural Science Foundation of China (Grant No. BK20180299), Jiangsu Agriculture Science and Technology Innovation Fund CX(19)3019, the Key Technology Research and Development Project of Jilin Province (Grant No. 20180201021NY).
-
XX designed the experiments and drafted the manuscript; QJ participated in designing the experiment; LQ and CX conducted part of the experiments; JW, JD, JL, WW, and WD conducted data analysis and assisted some of the experiments; ZD, NJ and RY conceived the study and finalized the manuscript.
-
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
-
The animal facility of Jilin University is fully accredited by the National Association of Laboratory Animal Care. All experimental procedures and animal (chicken) management procedures complied with the requirements of the Animal Care and Ethics Committees of Jilin University, China. The protocols for animal studies were also approved by the Animal Care and Ethics Committees of Jilin University (Approval numbers 201701256-01 for the chickens).














 DownLoad:
DownLoad: