HTML
-
The host innate immune system provides the first line of defense against microbial infection and mediates subsequent adaptive immunity. It is triggered by the recognition of intruding pathogens, components of which have been named pathogen-associated molecular patterns (PAMPs), by pattern recognition receptors (PRRs) expressed on the host cell (Cao 2016). PRR signaling results in the activation of interferon (IFN) regulatory factors (IRFs), nuclear factor κB (NF-κB), and other factors. Among IRFs, IRF3 is constitutively expressed in the cytosol in a latent form, because of its carboxy-terminal auto-inhibitory region (Sharma et al. 2003). In contrast, IRF7 is expressed at a low level and can be induced through the binding of IFNstimulated gene (ISG) factor 3 (ISGF3) to its promoter (Chen and Royer 2010). Upon stimulation, IRF3 and IRF7 undergo phosphorylation, leading to dimerization and translocation into the nucleus, which consequently results in the induction of IFN gene transcription (Yoneyama et al. 1998; Honda and Taniguchi 2006; Ikushima et al. 2013). In addition, IRF8 is involved in IFN expression by binding to the promoters of IFN genes (Tailor et al. 2007).
It is widely known that type Ⅰ IFN (IFN-α and IFN-β) and type Ⅲ IFN (IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4) play antiviral roles in response to viral infection (Schneider et al. 2014; Chen et al. 2018). For example, it was demonstrated that type Ⅲ IFNs exert inhibitory effect on porcine epidemic diarrhea virus (PEDV) replication, and that PEDV antagonizes its antiviral effects by interfering with IRF1 and NF-κB (Zhang et al. 2018). Following IFN expression, IFN-α and IFN-β bind with IFNAR, while IFN-λs bind with IFNL receptors (IFNLR), which stimulate the Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway (Chen et al. 2016). Phosphorylated STAT1 and STAT2 interact with IRF9 to form ISGF3. Importantly, ISGF3 translocates into the nucleus and binds the IFN-stimulated response element (ISRE), which initiates the transcription of hundreds of ISGs (Schneider et al. 2014).
These ISGs target different stages of the virus life cycle by suppressing viral entry, viral immune evasion, and viral gene transcription among other processes. For example, cholesterol 25-hydroxylase (CH25H), the myxovirus resistance protein (Mx) family, and IFN-induced transmembrane protein (IFITMs) family can restrict viral entry into cells (Liao et al. 2019; Ciminski et al. 2021; Zang et al. 2020). It was reported that human MxA can be induced by herpes simplex virus type1 (HSV-1) in the absence of IFN-α, which in turn facilitates viral replication (Ku et al. 2011). Recently, human MxB was identified as a potent inhibitor of HSV infection by restricting the uncoating of viral DNA (Crameri et al. 2018). CH25H, an integral element of cellular membranes, catalyzes the conversion of cholesterol to soluble 25-hydroxycholesterol (25HC), which is involved in antiviral responses against enveloped viruses, including pseudorabies virus (PRV) and influenza a virus (IAV) (Gold et al. 2014; Wang et al. 2017). Moreover, ISG15, a ubiquitin-like protein, inhibits IAV proliferation by perturbing viral release and viral protein translation (Yuan and Krug 2014). Interestingly, ISG15 can also serve as a receptor, binding intracellular proteins and regulating their functions. For example, ISG15 interacts with Ubl carboxy-terminal hydrolase 18 (USP18) to regulate IFN signaling and leucine-rich repeat-containing protein 25 (LRRC25) to induce the autophagic degradation of retinoic acid-inducible gene I (RIG-I) (Zhang et al. 2015; Du et al. 2018).
Alphaherpesviruses are large, double-stranded DNA viruses, which can cause latent infection and establish lifelong infection in animals and humans (Pomeranz et al. 2005). PRV is a porcine alphaherpesvirus that causes great economic losses to the pig industry. Recently, however, it was shown that PRV might also be a potential threat to humans, as evidenced by some cases of PRV infection with clinical symptoms of encephalitis (Ai et al. 2018; Li et al. 2020). Hence, investigation into host-PRV interaction is required to better understand its pathogenesis in both animals and humans. Previous studies have suggested that PRV infection could restrict innate immune responses, but the underlying mechanisms remain poorly known (Brukman and Enquist 2006a, 2006b). Until recently, PRV UL24 was shown to play a key role in host innate signaling by regulating NF-κB activation through the degradation of RelA (Wang et al. 2020). UL24 protein is a conserved protein in the herpesvirus family and is important for viral growth. In addition, HSV-1 UL24 is associated with the dispersal of nucleolin, which requires the endonuclease motif of UL24 (Lymberopoulos and Pearson 2007; Lymberopoulos et al. 2011). Moreover, HSV-1 UL24 was found to act as a regulator of DNA sensing signaling by suppressing NF-κB activation (Xu et al. 2017). Whether PRV UL24 shares these roles with other herpesviruses needs further investigation.
Among the ISG family, ISG20 is a 3'-5' exonuclease, which is localized predominantly in Cajal bodies, which are dense subnuclear structures of protein and RNA (Nguyen et al. 2001; Espert et al. 2006). Similarly, ISG20 has also been reported to block the replication of a range of viruses in previous studies. Hasan and colleagues found that ISG20 regulates hepatitis B virus (HBV) replication by selectively degrading N6-methyladenosine modified HBV transcripts (Imam et al. 2020). It has also been found that ISG20 restricts alphavirus growth by upregulating the expression of IFN-inducible antiviral proteins (Weiss et al. 2018). More recently, a study showed that ISG20 modulates translation without RNA degradation to perturb vesicular stomatitis virus (VSV) replication (Wu et al. 2019). Thus, ISG20 serves as an antiviral factor by degrading viral RNA or by enhancing IFN-mediated innate immune responses to restrict the virus life cycle.
Although studies on the pathogenesis of PRV have been performed, the role of ISG20 in PRV proliferation and the underlying mechanisms are not fully understood. In this study, we aimed to examine the role of ISG20 in PRV replication and investigate the underlying mechanisms by which ISG20 functions.
-
PK15 cells (CCL-33), HeLa cells (CCL-2), and HEK293T cells (CRL-11,268) were acquired from ATCC. They were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, D6429) supplemented with 10% fetal bovine serum (FBS; Gibco, 10,099,141) at 37 ℃ with 5% CO2. Cell transfection with plasmids was performed using Lipofectamine 3000 Reagent (Invitrogen, L3000015) and cell transfection with siRNA was performed using Lipofectamine RNAiMAX (Invitrogen, 13,778,150), according to the manufacturer's instructions. Porcine ISG20 siRNA and the control siRNA were synthesized by TSINGKE Biological Technology (Beijing, China) (Supplementary Table S1).
-
The PRV JS-2012 strain (GenBank: KP257591), the mutant virus, UL24-null PRV, and Sendai virus (SeV) are stored in our laboratory. PK15 and HeLa cells grown to approximately 90% confluence in 6-well plates were mockinfected with DMEM or infected with PRV at the indicated multiplicity of infection (MOI). HEK293T cells were stimulated by SeV at an MOI of 1. After incubation for 1 h at 37 ℃, cells were washed by PBS for 3 times to remove the unattached viruses and maintained in DMEM supplemented with 2% FBS at 37 ℃. Then PK15 and HeLa cells were collected at the indicated time points and HEK293T cells were collected at 16 h post-infection (hpi). The viral titers were calculated using Kaerber's method and exhibited as the 50% tissue culture infectious dose (TCID50) per milliliter.
-
Anti-ISG20 antibody (ab157477) was obtained from Abcam. Anti-Myc (SAB2108476) antibody was purchased from Sigma-Aldrich. Horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (SA00001-1), HRP-conjugated anti-rabbit IgG antibody (SA00001-2), and antiACTB/b-actin antibody (60,008-1) were purchased from Proteintech Group. Anti-HA-tag antibody (3724) was obtained from Cell Signaling Technology (CST). Anti-gB antibody was generated and stored in our laboratory. Poly(I: C) LMW (low molecular weight) was obtained from InvivoGen. The pcDNA3.1 (V87020) vector was obtained from Thermo Fisher Scientific. These recombinant plasmids used in this study were generated by homologous recombination using the ClonExpress II One Step Cloning Kit (Vazyme Biotech, C112-02).
-
Cells were washed three times with ice-cold PBS and lysed on ice in RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, 89,901) supplemented with Phosphatase Inhibitor Cocktail (Bimake, B15001) and Protease Inhibitor Cocktail (Bimake, B14001), according to the manufacturers' protocols. Briefly, the lysates were centrifuged and boiled for 5 min in 5× SDS-PAGE loading buffer. After that, 25 μg protein in each well was separated by 12% SDS-PAGE, and then transferred to 0.2-μm nitrocellulose Western blotting membranes (GE Healthcare, 10,600,001). Next, the membranes were blocked with tris-buffered saline (TBS) supplemented with 5% nonfat dry milk (BD, 232,100) and 0.1% tween-20 (Sigma-Aldrich, P1379) for 1 h at room temperature. After that, the membranes were incubated with the anti-ISG20 antibody, anti-gB antibody, anti-ACTB antibody, anti-HA antibody, or anti-Myc antibody. After incubation for 2 h at room temperature, the membranes were washed with TBS containing 0.1% tween-20 for 30 min. The membranes were then incubated with the HRP-conjugated anti-mouse IgG antibodies or the HRP-conjugated anti-rabbit IgG antibodies, at room temperature for 1 h. After washing with TBS containing 0.1% tween-20 for 30 min, the membranes were detected with chemiluminescence (Thermo Fisher Scientific, 34,580). The protein bands were quantified using ImageJ software (National Institutes of Health).
-
Viral DNA was prepared using the EasyPure DNA kit (TIANGEN Biotech, China). Total RNA was extracted from cells using Trizol reagent (15596-026, Thermo Fisher Scientific). For the analysis of reverse transcription (RT)- qPCR, cDNA was generated using the PrimeScriptTM RT Reagent Kit (Takara, RRO47A) and analyzed by real-time PCR with specific primers (Supplementary Table S1 and Table S2) using the ΔΔCT method with a LightCycler system (Roche, Switzerland). For the detection of PRV gB DNA copies, the sequences were as follows: ACTACGAGGACTACAGCTACG (gB-F), GTCACCCGCGTGC TGATC (gB-R), and ACGATCAGCACGCGGGTGACC (gB-probe).
-
The expression of porcine IFN-β from cell culture supernatants was analyzed with the Porcine Interferon β (IFN-β) ELISA kit (SEKP-0046, Solarbio), according to the manufacturer's instructions. Briefly, the cell supernatant was collected and added to microELISA stripplate wells to interact with the specific antibody. Then, HRP-conjugated antibodies were added and samples were incubated. After washing, tetramethylbenzidine (TMB) substrate solution was added to each well in the dark. Finally, the optical density (OD) was investigated spectrophotometrically at 450 nm when the stop solution was added. The concentration of IFN-β was determined by analyzing the OD of the samples and the standard curve.
-
All results represented three independent experiments. Data are shown as means ± standard deviations (SD) and calculated using the two-tailed Student's t-test or analysis of variance (ANOVA) based on at least three independent replicates. A P-value of < 0.05 was thought to be statistical significance for each test.
Cell Lines and Transfection
Viruses and Viral Infection
Antibodies, Plasmids, and Reagents
Western Blotting
Quantitative Real-time PCR
ELISA Assay
Statistical Analysis
-
We showed that both the mRNA and protein levels of ISG20 were increased in PRV-infected HeLa cells compared to those in mock-infected HeLa cells (Fig. 1A and 1B). We also showed that the ISG20 mRNA level was significantly enhanced in PRV-infected PK15 cells (Fig. 1C). However, we did not show the protein level of ISG20 in PK15 cells because this anti-ISG20 antibody is not effective against porcine ISG20. In addition, we found that the ISG20 mRNA level was significantly increased in HEK293T cells transfected with poly(I: C), and this was consistently increased with the increase of poly(I: C) concentration (Fig. 1D). These data suggest that both PRV infection and poly(I: C) treatment can upregulate the expression level of endogenous ISG20.
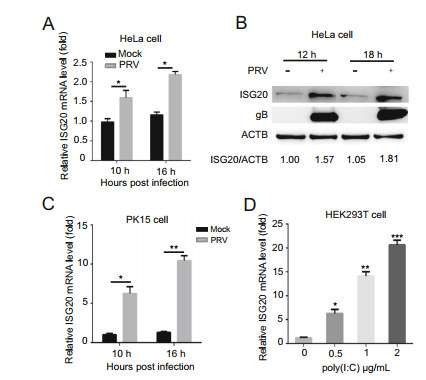
Figure 1. Endogenous ISG20 is induced by PRV infection and poly(I: C) treatment. A HeLa cells were mock-infected with DMEM or infected with PRV (MOI = 0.1) and collected at the indicated time points. The ISG20 mRNA levels were analyzed by real-time PCR. B The expression levels of ISG20, gB, and ACTB proteins in the same samples (A) were analyzed using Western blotting with the indicated antibodies. ACTB served as the control of sample loading. C PK15 cells were mock-infected or infected with PRV (MOI = 0.1) and harvested at the indicated time points. The ISG20 mRNA levels were analyzed by real-time PCR. D HEK293T cells were transfected with poly(I: C) using the indicated concentrations. After 12 h, the cells were collected and the ISG20 mRNA levels were analyzed by realtime PCR. The average levels from three independent experiments are plotted. The error bars stand for the ± S.D. from the mean. *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student's t test).
-
To investigate the ISG20-mediated effect on PRV replication, we performed ISG20 overexpression and knockdown assays. As shown in Fig. 2A, HA-ISG20 protein was expressed well in PK15 cells. Next, we employed real-time PCR to measure the PRV gB DNA copies and found that they were lower in PK15 cells in the presence of HAISG20 than in the control group (Fig. 2B). In addition, Western blotting analysis showed that the protein level of gB was decreased compared to that in the control group (Fig. 2C). Consistently, the viral titers were significantly reduced at the different time points, in contrast to those in the control group (Fig. 2D). Thus, we concluded that ISG20 overexpression negatively regulates PRV proliferation.
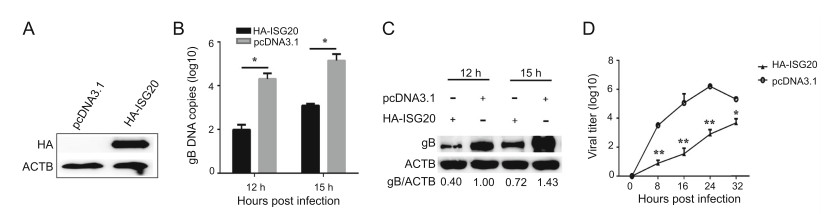
Figure 2. ISG20 expression suppresses PRV replication. A PK15 cells were transfected with the plasmids expressing HA-ISG20 (2 μg) or the empty vector (2 μg). The protein abundance of HA-ISG20 was analyzed by Western blotting using the indicated antibodies. B PK15 cells were transfected with plasmids expressing HA-ISG20 (2 μg) or the empty vector (2 μg). At 24 h post transfection, cells were infected with PRV (MOI = 0.1) and then collected at the indicated time points. PRV gB DNA copies were analyzed by real-time PCR. C PRV gB protein levels were detected with the indicated antibodies in the same samples (B). D The supernatants of PK15 cells described in (B) were collected and analyzed by TCID50 analysis for viral yield. The error bars stand for the ± S.D. from the mean. *P < 0.05, **P < 0.01 (two-tailed Student's t test).
-
Next, we employed an ISG20 knockdown assay to confirm the ISG20-mediated effect on PRV proliferation. Knockdown efficiency was determined using real-time PCR with specific primers (Fig. 3A). Next, we detected gB DNA copies and gB protein abundance in PK15 cells transfected with siRNA and infected with PRV. As expected, gB DNA copies in PK15 cells transfected with ISG20 siRNA were higher than those in the control group (Fig. 3B) and gB protein abundance was also increased compared to that observed in the control group (Fig. 3C). Consistently, the viral titers were increased in contrast to those in the control group (Fig. 3D). Together, these results indicate that ISG20 knockdown enhances PRV replication.
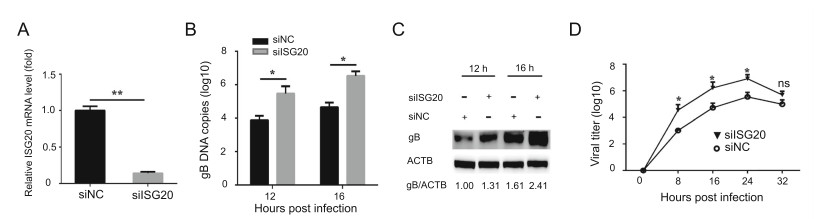
Figure 3. ISG20 knockdown promotes PRV replication. A PK15 cells were transfected with ISG20 siRNA and control siRNA. At 24 h post transfection, the cells were collected and analyzed for the knockdown efficiency by real-time PCR. B PK15 cells were transfected with siISG20 and siNC. At 24 h post transfection, cells were infected with PRV (MOI = 0.1) and then collected. PRV gB DNA copies were analyzed by real-time PCR. C PRV gB protein levels were detected with the indicated antibodies in the same samples (B). D The supernatants of PK15 cells described in (B) were collected and analyzed by TCID50 analysis for viral yield. The error bars stand for the ± S.D. from the mean. *P < 0.05, **P < 0.01 (two-tailed Student's t test).
-
Next, we investigated the underlying mechanisms by which ISG20 restricts PRV replication. Previous studies have shown that ISG20 exerts inhibitory effects on multiple RNA viruses by degrading viral RNA (Espert et al. 2003; Liu et al. 2017). It has also been reported that ISG20 enhances the IFN-mediated immune response or cooperates with dsRNA-dependent protein kinase (PKR) to mediate the IFN response (Jiang et al. 2008; Weiss et al. 2018). Here, we hypothesized that ISG20 expression might boost IFN-β expression during PRV infection. As a result, we found that ISG20 expression increased the mRNA levels of IFN-β during both mock and virus infections (Fig. 4A). In addition, the secretion of IFN-β was higher (Fig. 4B). Together, these data suggest that ISG20 expression leads to endogenous IFN-β upregulation.
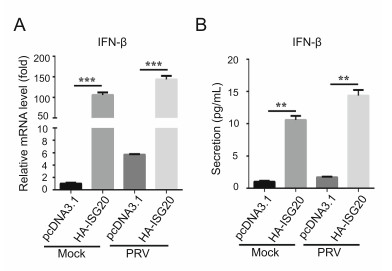
Figure 4. IFN-β expression is upregulated by ISG20 expression. A PK15 cells were transfected with the plasmids expressing HA-ISG20 (2 μg) or the empty vector (2 μg). Then cells were mock-infected or infected with PRV (MOI = 0.1) and collected at 12 h post infection. The mRNA level of IFN-β was detected by real-time PCR. B The supernatants of the cells in the same samples (A) were collected and analyzed for the secretion of IFN-β by ELISA assay. The error bars stand for the ± S.D. from the mean. **P < 0.01, ***P < 0.001 (twotailed Student's t test).
-
Type I IFN signaling inhibits virus replication through downstream gene products. Because we demonstrated that ISG20 induces IFN-β expression, we next investigated whether it could drive IFN signaling, enhancing downstream gene transcription. Real-time PCR analysis showed that ISG20 expression increased the transcription levels of some cytokines and ISGs. Specifically, we showed that the mRNA levels of IL-8 and TNF-α were increased during both mock and PRV infections (Fig. 5A and 5B). Similarly, the mRNA levels of MxA, ISG15, ISG56, and OASL were significantly enhanced (Fig. 5C-5F). Thus, ISG20 expression induces a subset of antiviral genes to suppress PRV replication.

Figure 5. ISG20 enhances transcription of IFN downstream antiviral genes. PK15 cells were transfected with the plasmids expressing HA-ISG20 (2 μg) or the empty vector (2 μg). At 24 h post transfection, cells were mockinfected or infected with PRV (MOI = 0.1). At 12 h post infection, cells were collected and analyzed for the mRNA levels of IL-8 (A), TNF-α (B), MxA (C), ISG15 (D), ISG56 (E) and OASL (F) by real-time PCR with specific primers. The error bars stand for the ± S.D. from the mean. **P < 0.01, ***P < 0.001 (two-tailed Student's t test).
-
It was previously shown that PRV infection suppresses the expression of some ISGs by inhibiting signal transducer and activator of transcription 3 (STAT3) phosphorylation (Brukman and Enquist 2006b). However, the underlying mechanisms remain poorly understood. Here, we considered the possibility that UL24 might regulate ISG20 expression. We found that the mRNA expression of endogenous ISG20 was significantly upregulated by RIG-I and poly(I: C) treatment. Notably, the mRNA levels of ISG20 were decreased in the presence of UL24 in HEK 293 T cells (Fig. 6A, 6B). Additionally, we found that the mRNA levels of ISG20 were higher in UL24-null PRVinfected cells than those in WT PRV infected PK15 cells (Fig. 6C). TCID50 analysis showed that the viral titers of WT PRV were higher than those of UL24-null PRV, indicating that UL24 plays an important role in PRV proliferation (Fig. 6D). Next, two mutants, pcDNA3.1-N-Myc (amino acids 1-90), and pcDNA3.1-C-Myc (amino acids 82-172) were generated, expressing two recombinant proteins at a size of approximately 10 kDa (Fig. 6E). Realtime PCR results showed that the N terminus (amino acids 1-90) was responsible for the suppression of ISG20 transcription (Fig. 6F). Together, these findings indicate that UL24 suppresses ISG20 transcription.
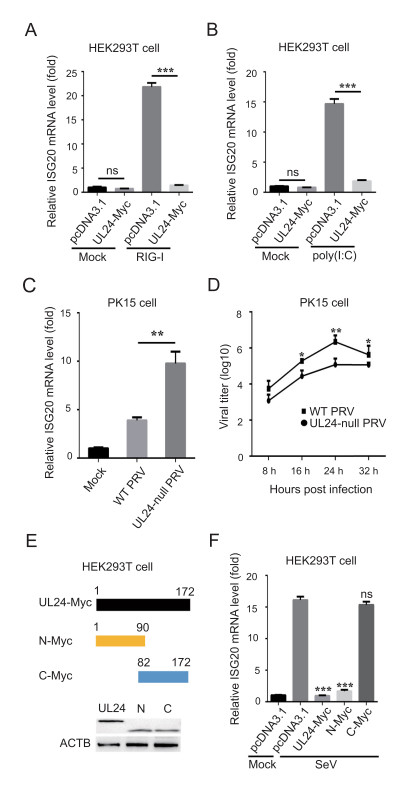
Figure 6. PRV UL24 suppresses ISG20 transcription. A HEK293T cells were transfected with the plasmids expressing UL24-Myc (1 μg) or the empty vector (1 μg) and/or RIG-I (0.2 μg) for 24 h. Total RNA was extracted and analyzed for ISG20 mRNA level by real-time PCR. B HEK293T cells were transfected with plasmids encoding UL24- Myc (1 μg) or pcDNA3.1 (1 μg) and stimulated by poly(I: C) (1 μg/ mL) for 12 h. Total RNA was extracted, and ISG20 mRNA levels were analyzed by real-time PCR. C PK15 cells were mock-infected with DMEM or infected with WT PRV (JS-2012) or UL24-null PRV (MOI = 0.1) for 12 h. Total RNA was extracted and ISG20 mRNA level was analyzed by real-time PCR. D PK15 cells were infected with WT PRV or UL24-null PRV and the supernatants were collected for the detection of viral titer by TCID50 analysis. E The schematic diagram of truncated UL24 proteins. HEK293T cells were transfected with the plasmids encoding UL24-Myc, or N-Myc, or C-Myc and collected for the analysis of expression by western blotting. F HEK293T cells were transfected with the plasmids expressing UL24-Myc (1 μg), N-Myc (1 μg), or C-Myc (1 μg) and stimulated by SeV for 16 h. Total RNA was prepared and ISG20 mRNA level was analyzed by real-time PCR. The error bars stand for the ± S.D. from the mean. *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student's t test).
Endogenous ISG20 Is Induced by PRV Infection and Poly(I: C) Treatment
Ectopic Expression of ISG20 Inhibits the Proliferation of PRV
ISG20 Knockdown Enhances the Proliferation of PRV
ISG20 Increases IFN-β Expression
ISG20 Enhances the Transcription of IFN Downstream Antiviral Genes
PRV UL24 Negatively Regulates ISG20 Transcription
-
Host innate immunity is the first line of defense against intruding pathogens. ISG is one of the most important molecules that exert potent inhibitory effects on viral replication (Chen et al. 2018). In this study, we determined the role of ISG20 during PRV infection. We found that ISG20 expression was upregulated at an early stage of PRV infection (before 24 hpi). However, it started to decline at 24 hpi (data not shown), indicating that PRV can counteract ISG20 expression at the late stage of infection. Similarly, ISG15 expression was increased at the early stage of PRV infection, but downregulated at the late stage (Liu et al. 2018). Next, we showed that ISG20 could negatively regulate PRV proliferation in PK15 cells using transient transfection and knockdown assays. Furthermore, ISG20 enhanced IFN-β expression and IFN downstream antiviral gene expression. Notably, we found that PRV UL24 protein served as a negative regulator of ISG20 expression, thus antagonizing its antiviral effects, which might partly account for the decline in ISG20 and ISG15 expression at the late stage of PRV infection. Interestingly, these results suggest that there is a race between host cell and the virus. On one hand, host cells respond to upregulate ISG20 expression and enhance IFN signaling, leading to the suppression of PRV infection. On the other hand, PRVencoded UL24 protein could restrict ISG20 transcription, thus leading to the evasion of host innate signaling and the establishment of successful infection. Since ISG20 is a potent inhibitor against viral infection, it could serve as a novel target to control PRV infection in animals. However, the host-virus interaction is very complicated, and animal infection with PRV requires further investigation in the future.
The role of ISG20 in vitro and in vivo is complicated, because ISG20 is reported to play various roles. For example, ISG20 expression leads to the radioresistant phenotype in clinically relevant radioresistant oral cancer cells (CRR-OCCs) and targeting ISG20 could result in efficient fractionated radiotherapy (RT) and chemoradiotherapy to remove cancer (Miyashita et al. 2020). It has also been reported that ISG20 promotes cell proliferation and metastasis and could serve as a potential biomarker and therapeutic target in clear cell renal cell carcinoma (ccRCC) (Xu et al. 2020). In addition, ISG20 facilitates local tumor immunity and results in poor survival in human glioma (Gao et al. 2019). These findings suggest that this protein plays an important role in cancer, and might serve as a potential therapeutic target against cancer. Interestingly, a long noncoding RNA (lncISG20) was identified to inhibit IAV infection in an ISG20-depedent manner (Chai et al. 2018).
Consistent with previous studies (Feng et al. 2018; Weiss et al. 2018), we showed that the overexpression of ISG20 inhibited PRV infection, whereas its knockdown promoted virus infection. Next, we investigated how ISG20 affects PRV infection. It was reported that ISG20 could upregulate IFN expression to exert antiviral effects on chikungunya and Venezuelan equine encephalitis viruses (Weiss et al. 2018). We found that the IFN-β mRNA levels were much higher in PK15 cells transfected with the HA-ISG20-encoded plasmids during PRV or mock infection. Next, we assessed IFN downstream signaling and found that the expression of some cytokines and ISGs was increased. Hence, we concluded that ISG20 enhances IFN signaling to inhibit PRV replication. Notably, ISG20 is a 3'-50 exonuclease enzyme, which can bind with viral RNA and degrade it (Espert et al. 2005). For example, Hasan and colleagues found that N6-methyladenosine (m6A)-modified HBV transcripts are selectively recognized and degraded by ISG20 (Imam et al. 2020). PRV UL54 protein is an RNA-processing protein that plays a key role in PRV replication at the early stage. Intriguingly, we discovered that ISG20 interacts with and colocalizes with UL54 protein (data not shown). Hence, we hypothesized that ISG20 might regulate viral RNA during PRV replication by interacting with UL54 protein. Nevertheless, further studies should be conducted to test this hypothesis.
UL24 protein is conserved in the herpesvirus family with similar localization patterns based on previous reports. HSV-1 UL24 was found to restrict NF-κB activation by impeding the nuclear translocation of p65 and p50 (Xu et al. 2017). It was also demonstrated that PRV UL24 perturbs NF-κB activation by degrading P65 (Wang et al. 2020). In the present study, our results revealed for the first time that PRV UL24 protein markedly inhibited the transcription of ISG20. Truncation experiments showed that the N terminus of UL24 (amino acids 1-90) was responsible for this inhibitory effect. These results provide new evidence that UL24 plays a critical role in antagonizing host innate immunity and helping PRV evade immune responses.
In conclusion, we showed that porcine and human ISG20 expressions were upregulated following PRV infection. The ISG20-mediated antiviral effects against PRV were confirmed by transient overexpression and knockdown assays in PK15 cells. Furthermore, ISG20 increased IFN-β expression and enhanced IFN signaling during PRV infection. However, PRV UL24 was found to inhibit the transcription of ISG20, thus helping the virus circumvent cellular innate immunity. Together, these results shed light on the critical role of ISG20 in restricting PRV proliferation and promote the understandings of PRV-host interplay.
-
We acknowledge the supports from the National Key Research and Development Program of China (2016YFD0500100), Shanghai Science and Technology Innovation Action Plan (17391901900), and Shanghai Municipal Agriculture Science and Technology Key Project (2016, 4-2).
-
XC and GT conceived and designed the experiments; XC, DS, SD, and HZ performed the experiments. NK, HZ, WT, and GL analyzed the data; XC wrote the manuscript and prepared the figures; XC, TS, and GT checked and finalized the manuscript. All authors contributed to the article and approved the submitted version.
-
The authors declare no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
















 DownLoad:
DownLoad: