HTML
-
Avian infectious bronchitis (IB) is an acute and highly contagious disease caused by infection with infectious bronchitis virus (IBV) (Zhao et al. 2019). It was first reported in the USA in 1931, and subsequently reported in various parts of the world, and seriously affects the development of the global poultry industry (Schalk et al. 1931). IBV belongs to the genus Coronavirus, family Coronaviridae, of the order Nidovirales (Mayo and Pringle 1998). The IBV genome consists of a single-stranded positive-sense RNA that encodes four structural proteins [spike (S), membrane (M), envelope (E), and nucleoprotein (N)] (Feng et al. 2017; Zhang et al. 2018, 2019). IBV primarily infects the respiratory tract, kidneys, and reproductive system of poultry, which can cause difficulty breathing and an enlargement of the kidneys (Cavanagh 2007). Moreover, infection in laying hens or breeders can cause false hens with a permanent decline in their egg production rate. In addition to infected chickens, it has been confirmed that the virus can also infect waterfowl and some ornamental birds (Chen et al. 2013). High genetic diversity, primarily in the S1 gene of IBV, has been demonstrated in different poultry-producing regions of the world (Bande et al. 2017). The high mutation and recombination rate, especially in the presence of host selection pressure, resulted in several IBV serotypes, making IBV one of the most difficult poultry diseases to control (Moreno et al. 2017).
Historically, the Massachusetts (Mass) and Connecticut (Conn) serotypes were the first isolates reported in the 1940s and 1950s, respectively (Schalk et al. 1931; Jungherr et al. 1956). The first IBV case in China was reported in the 1980s, and a growing number of IBV isolates have subsequently been reported (Zhao et al. 2016). A large number of IBV genotypes and variants have been isolated, of which the QX-like and Q1-like subtypes are the two most predominant genotypes in China. Recently, an increasing number of studies have shown that TW-type IBV has become the dominant strain in China (Gao et al. 2016; Yan et al. 2016). In Chinese mainland, the TW type IBV isolate is primarily TW I-type, and the TW II-type IBV has been isolated less frequently. Moreover, the TW-I type primarily causes nephritis lesions of an enlarged kidney (Yan et al. 2016). Although a large number of attenuated and inactivated vaccines based on Mass strains (H120, H52, W93, and Ma5) are widely used on farms in China, the protective efficacy is lacking (Feng et al. 2015). Unfortunately, there is no vaccine for the TW type strains currently. Therefore, the development of a live attenuated vaccine candidate based on TW-like IBV strain is required.
We previously isolated a TW I-type IBV strain, termed CK/CH/GD/GZ14 (Feng et al. 2017); however, we did not conduct an in-depth study on the pathogenicity and vaccine development of the strain. In this study, the pathogenicity of the CK/CH/GD/GZ14 strain was studied and an attenuated strain, CK/CH/GD/GZ14 p105, was obtained by a serial passage of the wild type IBV CK/CH/GD/GZ14 strain in SPF embryos. Further study showed that the live attenuated TW I-type IBV could provide favorable immune protection in chickens post-challenge with the IBV strain, CK/CH/GD/GZ14 p5. These results indicate that the live attenuated TW I-type virus may have the potential for developing IBV vaccine and provide a new vision for the clinical prevention and control of novel epidemic IBV.
-
The TW I-type IBV strain, CK/CH/GD/GZ14 was isolated in 2014 by our lab (Feng et al. 2017) and purified using the limiting dilution method (Zhang et al. 2018). CK/CH/GD/GZ14 p5 strain was purified with following procedure: Nine-day-old SPF chicken embryos were purchased from the SPF Experimental Animal Center of Guangdong Dahuanong Animal Health Co., Ltd. The virus stock were diluted into five gradients (from 10−5 to 10−9), and five SPF chicken embryos were inoculated for each gradient. After inoculation, the embryos were cultured in an incubator at 37 ℃ for seven days, the dead chicken embryos were discarded within 24 h, after 7 days, the allantoic fluid of chicken embryo was collected, and the chicken embryo was dissected, and the allantoic fluid which produced the maximum dilution multiple of dwarf embryo was selected to continue dilution and passage.
The passage procedure was: The purified strain, CK/CH/GD/GZ14 p5 strain, was diluted 100-fold with sterile physiological saline. Then, 0.1 mL of the virus dilution was inoculated into the allantoic fluid of ten day-old SPF chicken embryo and cultured in a 37 ℃ incubator for 72 h, after which the allantoic fluid was collected. During the later stages (40 generation), the time of death of the chicken embryos advanced due to the adaptation of the strain to the chicken embryo. The embryo was harvested 48 h after it was inoculated, and a continuous passage of 145 generations was performed. Viral purification was performed once every 30 passages [p35 (35th generation), p65 (65th generation), p95 (95th generation), p105 (105th generation), p120 (120th generation), and p135 (135th generation)].
-
A total of twenty 14 day-old chickens were purchased from the SPF Experimental Animal Center of Guangdong Dahuanong Animal Health Co., Ltd and were divided into a control group and an experimental group (n = 10 in each group). The chickens were kept in a positive pressure isolator and were free to eat and drink. The experimental group was challenged with CK/CH/GD/GZ14 (104.5 EID50/0.1 mL) per chicken by the nose and eye. The control group was inoculated with the same dose of PBS. The chickens were observed for morbidity characteristics daily for 15 days after challenge.
Hematoxylin and eosin (H&E) staining was used to evaluate the pathological characteristics of tissues of chickens infected with the CK/CH/GD/GZ14 strain. A histopathological section experiment was performed as previously described (Zhang et al. 2015).
-
A total of 90 ten-day-old SPF chickens were divided into nine groups, including a control group (10 chickens), p5 group (10 chickens), p70 group (10 chickens), p80 group (20 chickens), p95 group (20 chickens), and p105 group (20 chickens). The chickens in the experimental group were inoculated with allantoic fluid containing different doses of virus. For the p5 and p70 groups, the inoculation dose was 104.5 EID50/0.1 mL. Ten chickens in the p80, p95, and p105 groups were inoculated with 104.5 EID50/0.1 mL, whereas 10 chickens in the p80, p95, and p105 groups were inoculated with 103.5 EID50/0.1 mL. The virulence of different generations was evaluated by assessing the clinical symptoms and pathological changes of the tissues. The clinical symptoms and death of the chickens were recorded daily. Five days after the inoculation, three chickens were randomly selected from each group to evaluate the pathological changes in the trachea, lung, and kidney tissues.
-
The effectiveness of the attenuated virus was evaluated in the 95th and 105th generations. A total of 132 one-day-old SPF chickens were randomly divided into six groups (22 chickens per group): groups 1–4 were vaccinated with the 95th and 105th generations at the dose of 103.5 or 104.5 EID50 at day 10; group 5 was non-immunized (positive control group); and group 6 was the negative control group. At the age of 25 days, the chickens in the vaccinated and positive control groups were challenged with the 5th generation of CK/CH/GD/GZ14 at the dose of 104.5 EID50. The detailed grouping is shown in Table 1.
Group Generation of vaccinated strain Vaccination dose Generation of the challenge strain Challenge day Challenge dose 1 p95 103.5 EID50 p5 25 104.5 EID50 2 p95 104.5 EID50 p5 25 104.5 EID50 3 p105 103.5 EID50 p5 25 104.5 EID50 4 p105 104.5 EID50 p5 25 104.5 EID50 5 – – p5 25 104.5 EID50 6 – – – – – Table 1. Specific grouping for evaluation of vaccine effectiveness.
At 5 and 10 days after vaccination or 5 days and 10 days after challenge, three chickens from each group were sacrificed, and the tracheal, lung, and kidney tissues of important IBV-infected organs were collected. Detection of the viral copy number in tissues was assessed by qPCR after reverse transcription. Real-time PCR quantification of the viral load of each organ was conducted using standard curves as described previously (Chen et al. 2011). Briefly, two primers F: 5′-TTTGCTGGAACTTGTCTTGCAAGTATTAATG-3′ and R: 5′-CCTTCGTCTTTACTCTTGCTGATTGAAACAG-3′ were used to amplify a 600-bp IBV PCR product. Then the reaction product was analyzed on 1% agarose gels and ligated into a PMD-18-T vector (TaKaRa, Biotechnology, Dalian, China) to transform TOP10 competent cells. The positive clones were sequenced, and plasmids were extracted. The DNA concentration was calculated by measuring the absorbance at 260 nm. Using the DNA concentration, the plasmid copy number was calculated using the following formula: copy/μL = 6.02 × 1023 (copy/mol) × DNA concentration (g/μL)/MW (g/mol). Serial tenfold dilutions from 103 to 1010 copies of the purified plasmid were prepared in duplicate to produce a standard curve.
-
Three chickens from each group were sacrificed at day 5 after vaccination and day 5 after challenge and the trachea of each chicken was collected. The upper, middle, and lower sections of the tracheas were separated to observe the level of ciliary movement and shedding. The tracheal cilia activity was scored using a 0–4 scoring system. If the cilia in the whole trachea section display activity, the score is 0; if the cilia in the whole section of the trachea have approximately 75%–100% activity, the score is 1; if the cilia in the whole section of the trachea have approximately 50%–75% activity, the score is 2; if, in the whole trachea, 25%–50% of the cilia have activity, the score is 3; if less than 25% of the cilia in the whole trachea have an activity or the cilia have no activity at all, the score is 4. A significant difference analysis was performed between the experimental groups and the negative control group.
-
Throat and cloaca swabs of each group were collected after vaccination and after challenge at day 5, 10, and 15. The throat and cloacal swabs from the same chicken were mixed and shaken in 0.5 mL physiological saline (containing penicillin 8000 U and streptomycin 8000 U), repeatedly frozen, and thawed three times. The samples were centrifuged at 9300 ×g for 5 min. The supernatant was collected and 0.1 mL of the supernatant was inoculated into SPF chicken embryo. After six days, the chicken embryos were dissected to observe whether there were lesions characteristic of IBV, and the morbidity of each group was counted.
-
The clinical symptoms and death of the chickens were recorded daily following the challenge. The behaviour and clinical symptoms of the chickens were recorded daily. All of the surviving chickens in each group were subjected to the post-mortem examination, and pathological changes in the trachea, lungs and kidneys were examined by hematoxylin and eosin (H&E) staining. The morbidity and mortality of each group of chickens were assessed.
-
A total of 24 pairs of primers used to amplify the complete genome sequence of IBV were designed and synthesized by AuGCT DNA-SYN Biotechnology Co., Ltd. (Beijing, China) (Supplementary Table S1). Viral RNA extracted from the allantoic fluid of different generations was amplified using 24 primer pairs by reverse-transcription polymerase chain reaction (RT-PCR). The products of each RT-PCR were ligated to the cloning vector, pMD18-T (Takara, Japan), and transformed into competent cells. Positive clones were screened by PCR and sequenced by Shanghai Sang-gong Biological Engineering Technology & Services Co., Ltd. (Shanghai, China). The nucleotide sequences of each generation were sequenced and assembled into a complete genome sequence respectively. The genome sequences were compared with that of the CK/CH/GD/GZ14 strain using DNAstar software.
-
Statistical analyses were performed with GraphPad Prism software and expressed as means and standard deviation. The statistical significance of data was calculated with a one-way analysis of variance (ANOVA) between the experimental groups. Significant differences are indicated as * P < 0.05 and ** P < 0.01.
The Serial Passage of the TW I-Type IBV CK/CH/GD/GZ14 Strain
Pathogenicity Study of the TW I-Type IBV Strain, CK/CH/GD/GZ14
Evaluation of the Attenuated Effects of CK/CH/GD/GZ14 with Different Generations
Evaluation of Vaccine Efficacy Based on the Viral Load in the Organs
Evaluation of Vaccine Efficacy Using the Tracheal Cilia Stagnation Experiment
Evaluation of Vaccine Efficacy Based on the Virus Shedding
Evaluation of Vaccine Efficacy Based on Clinical Morbidity and Histopathological Sections
Complete Gene Sequencing Analysis of Different Generations of CK/CH/GD/GZ14 Viruses
Statistical Analysis
-
The 14 day-old chickens in the experimental group were infected with the CK/CH/GD/GZ14 strain (n = 10). On the sixth day post-infection, one chicken exhibited sneezing, listlessness, and huddling. Chicken death began on the seventh day post-infection. The post-mortem examination revealed tracheal congestion, massive urate deposition in the heart and ureter, and renal enlargement with variegated appearance, whereas the trachea, heart, ureter and kidney in the negative control group were unaffected (Fig. 1). The follow-up record was continued until 15 days post-challenge. A total of eight chickens died, thus the mortality rate of CK/CH/GD/GZ14 strain was 80%. At the end of the experiment, all the chickens in experimental group displayed obvious renal swelling. However, the chickens in the negative control group had no sneezing, listlessness, and huddling, and the kidneys were normal after dissection. The results showed that the TW I-type IBV CK/CH/GD/GZ14 strain had strong pathogenicity to chickens, especially causing kidney enlargement, with the morbidity rate of 100%.
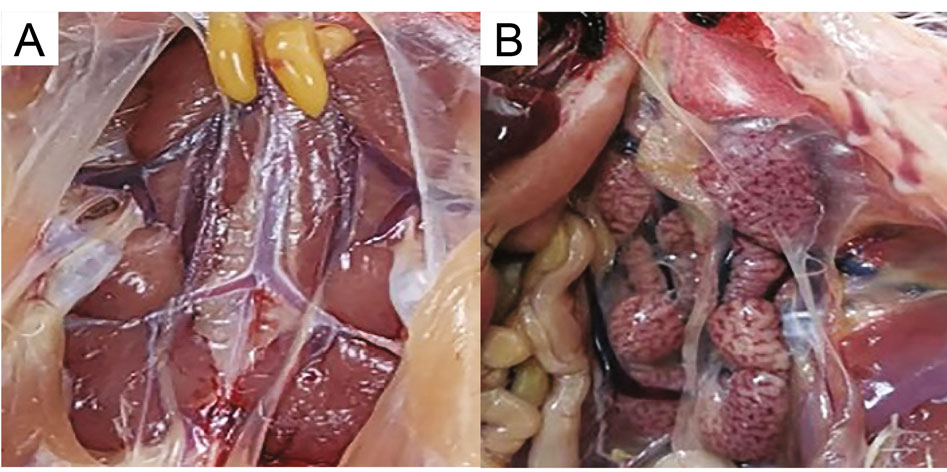
Figure 1. Representative images of severe kidney enlargement and congestion induced by CK/CH/GD/GZ14 to SPF chickens. Fourteen-day-old SPF chickens were infected with CK/CH/GD/GZ14 (104.5 EID50/0.1 mL per chicken) or PBS via the nose and eye. The kidney was macroscopically examined at 7 days post-challenge. Representative images from the uninfected group and infected SPF chickens are shown in A and B respectively. A Normal kidney of SPF chickens inoculated with PBS in the negative control group. B The enlarged and mottled kidney of SPF chickens infected with CK/CH/GD/GZ14 (black arrow).
-
Our results showed that CK/CH/GD/GZ14 strain could cause 100% morbidity and 70% mortality in infected chicken embryos. With the increase in passage generation on SPF embryos, the virulence of the virus against 10 day-old SPF chickens gradually weakened, and the morbidity rate of the 95th generation (p95) and the 105th generation (p105) was 20% after infection, which was milder than the virulence associated with the 5th generation.
As shown in Table 2, compared with the p80/103.5 EID50 group, the p80/104.5 EID50 group had high morbidity and mortality in SPF chickens, whereas none of the p80/103.5 EID50 group died. The p95 and p105 groups had fewer diseased chickens than the p80, p70, and p5 groups. The p95 and p105 groups did not have any dead chickens and the symptoms were milder than those in the p5 group were.
Group Generation/Vaccination dose Morbidity Mortality 1 p5/104.5 EID50 10/10 7/10 2 p70/104.5 EID50 5/10 3/10 3 p80/104.5 EID50 3/10 3/10 4 p80/103.5 EID50 3/10 0/10 5 p95/103.5 EID50 2/10 0/10 6 p95/104.5 EID50 2/10 0/10 7 p105/103.5 EID50 2/10 0/10 8 p105/104.5 EID50 2/10 0/10 Negative control – 0/10 0/10 Table 2. The morbidity and mortality of chickens after vaccination of CK/CH/GD/GZ14 strain of different generation.
As shown in Fig. 2, in the p5/104.5 EID50 infected group, the tracheal lamina propria displayed edema, the mucosal upper layer had blood cell shedding; the pulmonary bronchial interstitium showed a large number of inflammatory cell hyperplasia; the alveolar cavity exhibited inflammation and was blocked, and there was a large amount of inflammatory cell infiltration in the kidney. However, the lung and kidney tissues of the p95/103.5 EID50-, p95/104.5 EID50-, p105/103.5 EID50-, and p105/104.5 EID50-infected groups were normal; only the trachea in the p95/103.5 EID50 and p95/104.5 EID50 groups had edema of the lamina propria. According to the histopathological sectioning results, the pathogenicity of the CK/CH/GD/GZ14 strain in the trachea, lungs, and kidneys gradually weakened with the increasing number of passages, and pathogenicity was significantly reduced when passaged to p105.
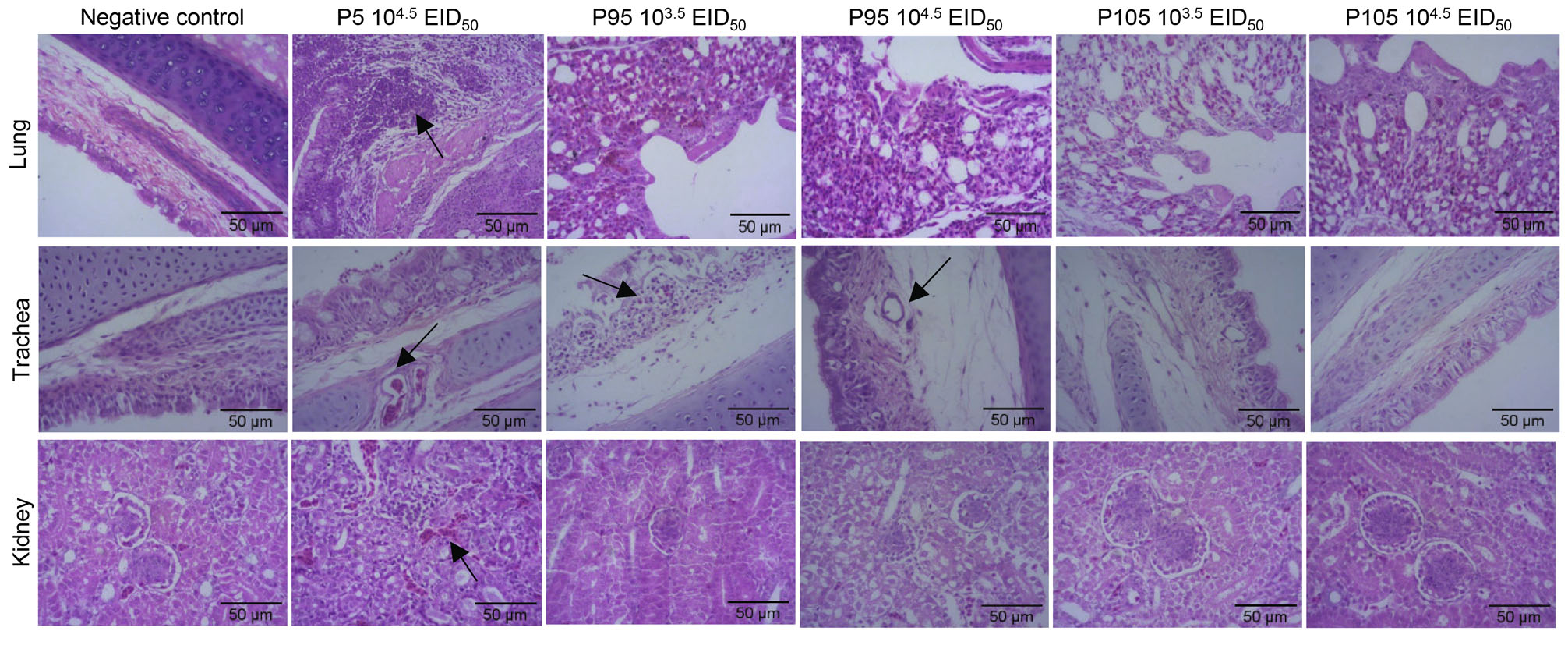
Figure 2. Histopathological changes in the lungs, trachea, and kidneys of SPF chickens infected with CK/CH/GD/GZ14 of different generations. Ten-day-old SPF chickens were infected with 104.5 EID50/0.1 mL 5th (p5), 103.5 and 104.5 EID50/0.1 mL 95th (p95) and 105th (p105) generations of CK/CH/GD/GZ14 passaged in SPF chicken embryos. After infection for 5 days, the lungs, trachea, and kidneys were examined by H&E staining (200 ×). The chickens in the negative control group were inoculated with 0.1 mL PBS. The histopathological changes were denoted by arrows. In the p5/104.5 EID50 infected group: a large number of inflammatory cells proliferated and infiltrated in the bronchiolar interstitium and a large number of inflammatory substances were blocked in the alveolar cavity; Edema of lamina propria and exfoliation of blood cells in the upper layer of mucosa; Congestion and infiltration of a large number of inflammatory cells. In the p5/103.5 EID50 infected group, the trachea showed the edema of lamina propria and infiltration of inflammatory cells in mucosal layer. In the p5/103.5 EID50 infected group, the structure of trachea is normal while the lamina propria is edema for the trachea.
-
In the non-immunized group (positive control group), the chickens exhibited sneezing, listlessness, and huddling on the fourth day after challenge with the CK/CH/GD/GZ14 p5 strain. However, the chickens vaccinated with the CK/CH/GD/GZ14 p95 and p105 strains appeared normal following the challenge. All chickens were sacrificed on day 15 post-challenge, and two chickens in the p95 group displayed serious renal swelling, while two chickens in the group vaccinated with the CK/CH/GD/GZ14 p105 strain exhibited moderate renal enlargement. Only four chickens in the non-immunized group (n = 22) remained at 15 days post-challenge, with kidney enlargement (Fig. 3). Furthermore, the lung, trachea, and kidneys of the remaining chickens in the vaccinated groups were normal, while lungs of chickens in the positive control group showed hemorrhaging and extensive shedding of bloody tissue. Large amounts of bloody and inflammatory substances were observed in the upper layer of the trachea, and the kidney showed a large degree of inflammatory cell proliferation and infiltration (Fig. 4).
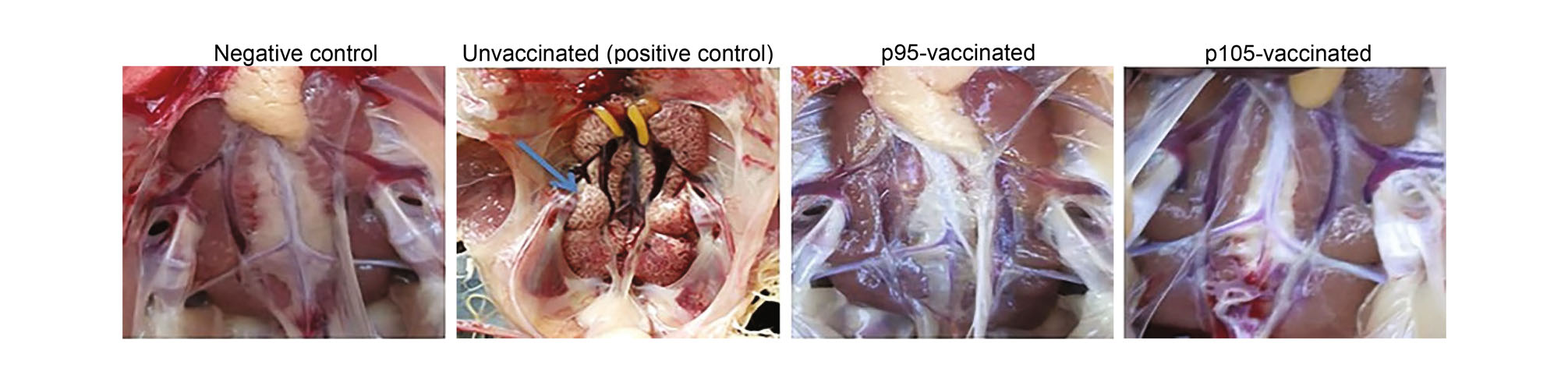
Figure 3. Representative images of kidneys in p95-, p105-vaccinated chickens following challenge. A dose of 104.5 EID50/0.1 mL of the 95th-generation strain (p95) and the 105th-generation strain (p105) was used for vaccination in 10 day-old SPF chickens, and a dose of 104.5 EID50/0.1 mL of the 5th-generation strain (p5) was used for viral challenge. Representative images of the kidneys from unvaccinated (positive control), p95-vaccinated, p105-vaccinated chickens after challenge were shown. The enlarged and mottled kidney in unvaccinated group were indicated by blue arrows, while the kidneys in vaccinated (p95 or p105) and challenged chickens were normal.

Figure 4. Histopathological changes in the lungs, trachea, and kidneys of 10 day-old chickens in different vaccinated groups at 15 days post-challenge with CK/CH/GD/GZ14 (p5). SPF chickens were vaccinated with 103.5 and 104.5 EID50/0.1 mL CK/CH/GD/GZ14 p95 or p105 strains and then challenged with the 5th-generation strain (p5) at the dose of 104.5 EID50/0.1 mL at 15 days post-vaccination. The lungs, trachea, and kidneys were examined by hematoxylin and eosin (H&E) staining (200 ×) at 15 days post-challenge. The lung, trachea, and kidneys of the remaining chickens in the vaccinated groups were normal. The histopathological changes in the positive control group were denoted by arrows: Congestion and bleeding, with a large amount of bloody material shedding in lung. Edema of lamina propria of mucous membrane and exfoliation of a lot of bloody and inflammatory substances in the upper layer of trachea. Proliferation and infiltration of a large number of inflammatory cells in kidney.
The challenge experiment results showed that the CK/CH/GD/GZ14 strain of the 95th and 105th generations could provide better protection against the CK/CH/GD/GZ14 strain, with a relative protection rate of 80% (Table 3). There was no significant difference in the tracheal ciliary motility between the p105/p95 (103.5 EID50)-vaccinated group and negative control group at five days post-vaccination (P > 0.05) (Fig. 5A). However, the p95 (104.5 EID50) group showed ciliary movement stagnation and ciliary shedding, and the difference was extremely significant compared with the negative control group (P < 0.01), while there was no significant difference in the tracheal ciliary motility between the p105 (104.5 EID50)-vaccinated group and the non-vaccinated control group (P > 0.05), illustrating that p105 generation is more suitable for vaccine development. Furthermore, the difference of tracheal cilia activity between the vaccinated/challenged groups and the unvaccinated/challenged positive control group was extremely significant at 5 days post-challenge (P < 0.01) (Fig. 5B).
Group Generation of vaccinated strain Vaccinated dose Generation of the challenge strain Anatomy results Morbidity (n = 10 in each group) Protection rate 1 p105 103.5 EID50 p5 1 chicken showed mild kidney enlargement and 1 chicken showed kidney enlargement 2/10 80% 2 p105 104.5 EID50 p5 1 chicken showed mild kidney enlargement, 1 chicken showed kidney enlargement and yolk malabsorption 2/10 80% 3 p95 103.5 EID50 p5 2 chickens showed kidney enlargement 2/10 80% 4 p95 104.5 EID50 p5 2 chickens showed kidney enlargement 2/10 80% 5 – – p5 10 chickens showed kidney enlargement and urate deposition 10/10 0 6 – – – The kidneys of all chickens were normal 0 – Table 3. Protective efficacy of the attenuated virus CK/CH/GD/GZ14 of different generation to 10-day-old SPF chickens against challenge with the CK/CH/GD/GZ14 virus of the 5th generation.
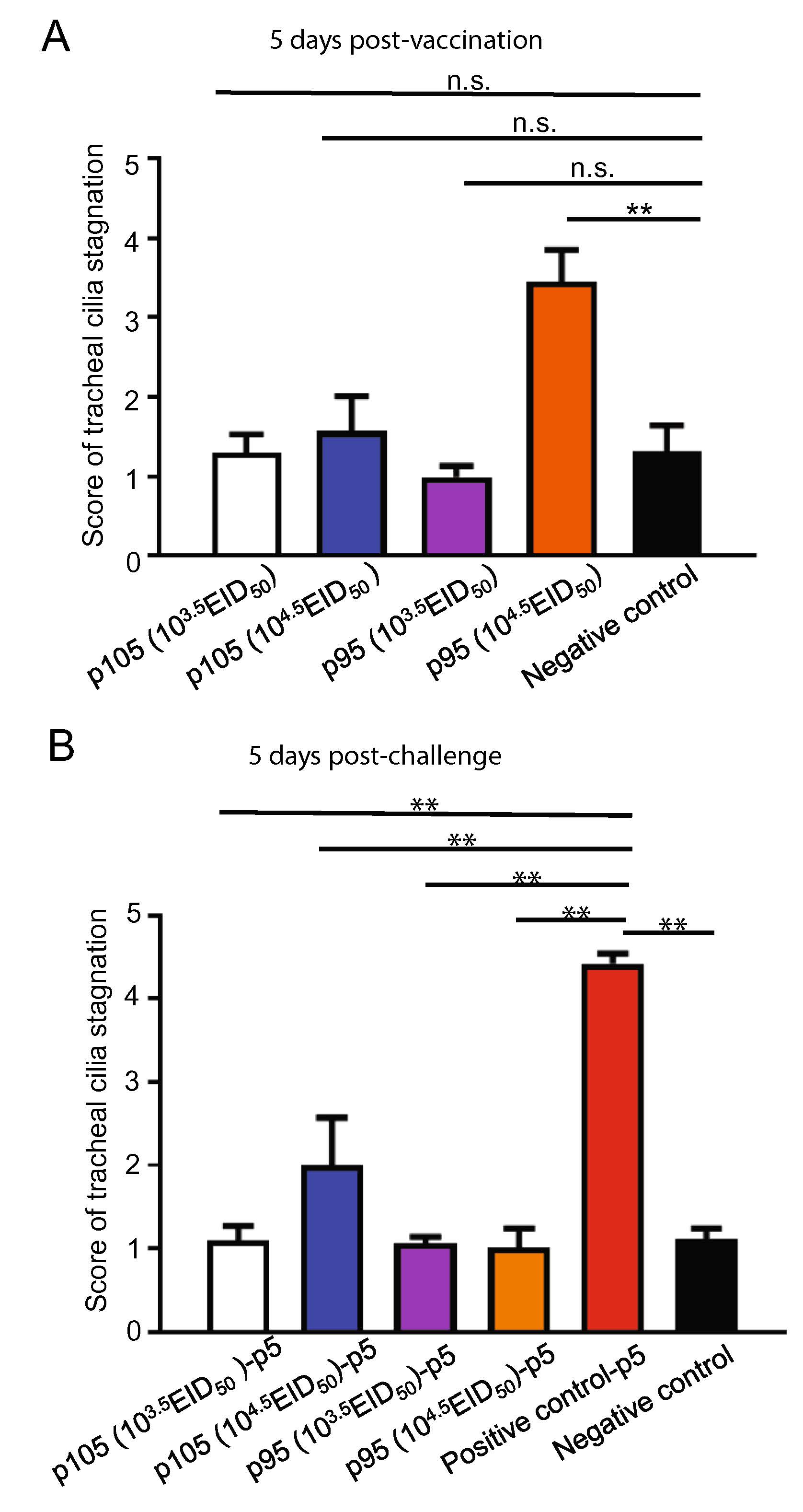
Figure 5. The cilia stagnation of chickens in the different vaccinated and vaccinated/challenged groups. A Ten-day-old SPF chickens were vaccinated with CK/CH/GD/GZ14 strains of different generations using different inoculation doses. At five days post-vaccination, the ciliary movement stagnation in different groups was analyzed. The p105-vaccinated group was better than the p95-vaccinated group according to the score of tracheal cilia stagnation. B Ten-day-old SPF chickens were vaccinated with CK/CH/GD/GZ14 strains of different generations using different inoculation doses. After 15 days post-vaccination, the chickens were challenged with CK/CH/GD/GZ14 of the 5th generation for 5 days, and then, the ciliary movement stagnation in different groups was further analyzed. A dose of 104.5 EID50/0.1 mL was used for viral challenge. There was no significant difference for the score of tracheal cilia stagnation between the p95-vaccinated/challenged and p105-vaccinated/challenged groups. ** denotes P < 0.01, n.s. denotes not significant, compared with the control group.
As shown in Supplementary Table S2, after vaccination with the same dose of virus, the rate of virus shedding in the p105-vaccinated group was lower than that of the p95 group. At 10 days post-challenge, the virus shedding rate of the throat swab in the p105-vaccinated group was significantly lower than that in the p95-vaccinated group, suggesting that with the increasing number of passages, the ability of the attenuated strain to block virus shedding gradually enhanced.
The CK/CH/GD/GZ14 virus was detected in the trachea, lungs, and kidneys in the p95 and p105 vaccinated groups at 5 and 10 days post-vaccination, of which the highest number of viral RNA copies was observed in the trachea (Fig. 6A, 6B). The viral RNA copies in the lungs, trachea, and kidneys of the p105-vaccinated group were significantly lower than those in the same dose p95-vaccinated group post-challenge (Fig. 6C, 6D). Collectively, these data suggest that p105 providing efficient protection from infection with the TW I-type CK/CH/GD/GZ14 virus.

Figure 6. Viral RNA copies of the major organs (kidney, lung and trachea) at 5 and 10 days after p95 and p105 vaccination or at 5 and 10 days post-challenge with CK/CH/GD/GZ14 of the 5th generation in different vaccinated groups. A, B The viral RNA copies were detected in the trachea, lungs and kidneys at 5 (A) or 10 (B) days post-vaccination. C, D The viral RNA copies were tested in the trachea, lungs and kidneys of p95- and p105vaccinated chickens followed by challenging with CK/CH/GD/GZ14 of the 5th generation at day 5 post-challenge. *denotes P < 0.05, ** denotes P < 0.01, compared with the control group.
-
The complete gene sequences of the p50, p80, p95, p105 and p120 strains were found to have nucleotide point mutations at multiple positions compared with p5. There were 11 nucleotide point mutations in p50 which were highly stable and continued to p120. There were six mutations generated during passaging from the p50 generation to the p80 generation, as well as one nucleotide insertion and one nucleotide deletion in the 5′ UTR region. Furthermore, three mutations occurred from p80 to p95 passaging, and one mutation generated from p95 to p105 passaging. Nucleotide mutations, insertions, and deletions ultimately resulted in the insertion and replacement of 19 amino acids in the entire CK/CH/GD/GZ14 genome. Among the 19 amino acid mutations, seven amino acid mutations occurred in the 1a gene (V957F, A1209S, W2249R, Y2491H, Q2707R, Q3500K and A3929V); four amino acid mutations occurred in the S1 gene (P118L, T179A, V387F, and R411L); three amino acid mutations occurred in the S2 gene (A222V, S345F, and S468F); two amino acid mutations occurred in the N gene (P145L and A403P); and one amino acid mutation occurred in each of the 1b (A423S), E (R70I), and 5a (E32K) genes (Table 4), indicating that low pathogenicity and good antigenicity may have a relationship with the stable amino acid point mutation of 1a, S1, S2, N, 1b, E, and 5a genes, as well as nucleotide insertions and deletions in the 5′ UTR region.
Gene Nucleotide position p5–p50 p50–p80 p80–p95 p95–p105 p105–p120 Amino acid position Amino acid insertion and replacement 5'-UTR 16 – insert A – – – – – 5'-UTR 18 – A → T – – – – – 5'-UTR 21 – deletion T – – – – – 1a 2854 G → T – – – – 957 V → F 1a 3625 G → T – – – – 1209 A → S 1a 6844 – T → C – – – 2249 W → R 1a 7471 T → C – – – – 2491 Y → H 1a 8280 A → G – – – – 2707 Q → R 1a 10, 498 C → A – – – – 3500 Q → K 1a 11, 457 T → A – – – – 3820 Silent 1a 11, 786 C → T – – – – 3929 A → V 1b 1414 – G → T – – – 423 A → S E 209 – – G → T – – 70 R → I S1 353 C → T – – – – 118 P → L S1 535 – A → G – – – 179 T → A S1 1159 – G → T – – – 387 V → F S1 1232 – G → T – – – 411 R → L S2 665 – – – C → T – 222 A → V S2 1034 C → T – – – – 345 S → F S2 1403 C → T – – – – 468 S → F 5a 94 – – G → A – – 32 E → K N 434 – – C → T – – 145 P → L N 1207 G → C – – – – 403 A → P Table 4. Summary of nucleotide and amino acid mutations during CK/CH/GD/GZ14 passage.
Assessing the Pathogenicity of the CK/CH/GD/GZ14 Strain
Assessing the Safety of the Different Generations of the CK/CH/GD/GZ14 Strain
Assessment of Vaccine Efficacy of the 95th Generation (p95) and the 105th Generation Histopathological Sections
Complete Gene Sequencing Analysis of Different Generations of the CK/CH/GD/GZ14 Isolate
-
Although a large number of live and inactivated IBV vaccines based on different genotype strains are widely used worldwide, IB outbreaks continue to occur sporadically due to high mutation rate and poor cross-protective effects of the different IBV strains (Cook et al. 2001; Liu et al. 2009b; Xue et al. 2012; dos Santos et al. 2019). Recently, an epidemiological survey of IBV in China has shown that the TW-type IBV strain has become a widespread genotype strain, and often recombines as a major parental strain with the 4/9- and QX-type IBV strains (Huang et al. 2004; Chen et al. 2010; Ma et al. 2012; Gao et al. 2016; Feng et al. 2017). Currently, there is no effective vaccine against TW I-type strains. Therefore, the development of an effective vaccine candidate against TW I-type IBV is required as an important measure to prevent TW-I IBV from causing major economic losses to the poultry industry.
The continuous passage of chicken embryos is a common method used for the preparation of attenuated IBV vaccines (Yachida et al. 1979; Cook et al. 2012). Normal temperature, heat treatment, and cold adaptation passaging are three commonly used methods for viral attenuation (Jackwood et al. 2010). In this study, the TW I-type IBV strain, termed CK/CH/GD/GZ14, gradually adapted to chicken embryos, and the virulence in chicken embryos was also significantly enhanced; however, the pathogenicity towards chickens decreased with the continuous passage. In particular, after 80 passages, the infected chickens no longer died. Histopathological observations confirmed that the pathogenicity of the virus in the trachea, lungs, and kidneys was significantly weakened after the administration of p95. In particular, when passaged to p105, the microstructure of each tissue was the same as that of the control group.
Vaccine efficacy based on clinical morbidity and histopathological sections was evaluated in chickens in response to CK/CH/GD/GZ14 strain challenge, showing that the attenuated CK/CH/GD/GZ14 strain at the 95th and 105th generations could provide good protection against infection with the CK/CH/GD/GZ14 strain. Recent studies have shown that IBV vaccine candidate strains should pay special attention to the results of cilia stagnation and virus shedding experiments (Jackwood et al. 2010; Bande et al. 2015). Hence, these two experiments were also carried out in this study. Results showed that the cilia movement in the trachea was normal with a low-dose vaccination of CK/CH/GD/GZ14 in the 95th generation, whereas at a high dose, the lower end of the tracheal cilia appeared to be in stagnation and accompanied by shedding. However, the degree of tracheal ciliary movement was normal in all segments in the 105th-generation-vaccinated groups. Moreover, after challenge with the 5th generation of CK/CH/GD/GZ14, the scores of tracheal cilia stagnation in the p105- and p95-vaccinated groups were significantly lower than that of the positive control group; moreover, at the same dose of vaccination, the rate of virus shedding in the p105-vaccinated group was lower than that of the p95 group, in particular, the rate of virus shedding in the throat swabs at 10 days post-challenge, suggesting that the ability of attenuated strains to block virus shedding gradually improved with the increasing number of passages in p105.
Measuring the viral load in different organs after viral challenge is a key indicator of vaccine effectiveness (He et al. 2019). Experiments performed to determine the viral load in different organs further confirmed that the number of viral copies in the different organs of chickens challenged with the 5th-generation CK/CH/GD/GZ14 in the p95- and p105-vaccinated groups were significantly lower than that of the group only challenged with the CK/CH/GD/GZ14 of the 5th generation. Moreover, the viral load in different organs of the p105-vaccinated group was lower than that in the p95-vaccinated group at the same dose vaccination, which demonstrated that the p105-vaccinated group had better immunogenicity and could effectively reduce the replication of the TW I-type IBV virus in the chicken body.
Since the IBV S1 protein can induce hemagglutination inhibitory and virus-neutralizing antibodies as well as playing an important role in viral tissue tropism, several studies on the pathogenicity of IBV are restricted to the S1 gene (Casais et al. 2003), but further research on IBV has shown that the S1 protein is not the key factor determining the pathogenicity of IBV. One report in 2007 clearly showed that one or more of the 15 proteins contained in 1a and 1b genes determine the pathogenicity of IBV. Similarly, the latest research demonstrated that the replicase 1a gene plays a critical role in the pathogenesis of avian coronavirus infectious bronchitis virus (Cavanagh 2007; Zhao et al. 2020). In the same year, it was reported that the partial deletion of the 3′ UTR gene may be related to viral pathogenicity (Huang and Wang 2007). In 2018, Laconi reported that either the 3a, 3b, 5a, or 5b gene from IBV induces an attenuated phenotype both in vitro and in vivo (Laconi et al. 2018). By comparing changes in the viral gene before and after the attenuation of IBV passage, we found that a stable point mutation was found in the nucleotides of p50, p80, p95, and p105 compared with the p5 generation, which finally caused a stable substitution of 19 amino acids (aa) in the complete CK/CH/GD/GZ14 genome; those amino acid point mutations were located in the 1a, S1, S2, N, 1b, E, and 5a genes. Furthermore, we found that nucleotide insertions and deletions occurred in the 5′ UTR region. Since it has been reported that the 1a, 1b, 3′ UTR, 5a, 5b, 3a and 3b genes of IBV are all closely associated with the pathogenicity of IBV(Cavanagh 2007; Huang and Wang 2007; Laconi et al. 2018; Zhao et al. 2020), our study found that aa mutations in both 1a, 1b, and 5a genes were present in the genome of the attenuated IBV strain CK/CH/GD/GZ14, thus, again, suggesting that the aa mutations in 1a (V957F, A1209S, W2249R, Y2491H, Q2707R, Q3500K, and A3929V), 1b (A423S), and 5a (E32K) genes play an important role in the low pathogenicity of the attenuated IBV strain CK/CH/GD/GZ14. Interestingly, aa mutations in the N (P145L and A403P) and E (R70I) genes and nucleotide deletions and insertions in the 5′ UTR were found in the genome of the attenuated IBV strain CK/CH/GD/GZ14, indicating that the N, E and 5'UTR may be also closely related to the attenuation of IBV. Thus, we speculate that multiple sites, genes alone, or a combination of genes can affect the virulence and pathogenicity of IBV (Huang and Wang 2007; Liu et al. 2009a; Feng et al. 2015; Zhao et al. 2015; Huo et al. 2016). Protective immunity is associated with the major structural protein, spike (S) glycoprotein, the majority of neutralizing antibodies are targeted towards the S1 subunit (Ignjatovic and Galli 1994; Keep et al. 2020). Another study demonstrated that the nucleocapsid N protein of IBV plays a key role in the induction of protective immunity (Boots et al. 1991). In this study, we found that aa mutations in both S and N genes, indicating that these aa mutations in the S gene (S1 protein: P118L, T179A, V387F, and R411L; S2 protein: A222V, S345F, and S468F) and N gene (P145L and A403P) may be closely related to the good immunoprotective efficiency of the attenuated IBV strain CK/CH/GD/GZ14. However, the mechanism associated with the relationship between mutations and the pathogenicity of these amino acids requires further verification.
In summary, an attenuated strain CK/CH/GD/GZ14 was obtained after the serial passage of SPF chicken embryos to 105 generations. The attenuated strain of the 105th generation could resist the attack of CK/CH/GD/GZ14 of the 5th generation in chickens, and the relative protection rate could reach 80%. In addition, the 105th-generation attenuated strain had no significant effect on tracheal ciliary movement in chickens, and the viral RNA copy number in the trachea, lungs, and kidneys was significantly lower than that in the challenge group of CK/CH/GD/GZ14 in the 5th generation. There were 19 amino acid insertions and substitutions in the whole genome of the attenuated strain, concentrated in the S1, S2, N, 1B, E, and 5a genes. The insertion and substitution of these 19 amino acids may be the reason for the reduced virulence and good immunogenicity of CK/CH/GD/GZ14. Therefore, the attenuated strain of the 105th generation can be used as a vaccine candidate.
-
This study was supported by the Key Research and Development Program of Guangdong Province (2020B02022 2001), the Construction of Modern Agricultural Science and Technology Innovation Alliance in Guangdong Province (2020KJ128), the Natural Science Foundation of Guangdong Province (2019A151501 2006), the National Natural Science Foundation of China (31902252), the Special Project of National Modern Agricultural Industrial Technology System (CARS-41), the National Modern Agricultural Industry Science and Technology Innovation Center in Guangzhou (2018kczx01), the National Key R&D Program of China (2017YFD050 2001) and the Creation of a Triple Chimeric Vaccine (rIBV-ND-H9) Using Avian Infectious Bronchitis Attenuated D90 as a Vector (2017KZDM008).
-
XZ conducted the experiments, analyzed the data and wrote the manuscript. TC conducted the experiments. SC, YN, ZX and KF analyzed part of the data. HZ checked and finalized the manuscript. QX designed the experiments, modified the manuscript, and supervised the whole work. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
All experiments were carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The use of animals in this study was approved by the South China Agricultural University Committee of Animal Experiments (approval ID: SYXK2019-0136).
















 DownLoad:
DownLoad: