HTML
-
Coxsackievirus B1 (CVB1), belonging to the Enterovirus genus in the Picornaviridae family, is a major member of the Coxsackievirus B (CVB) species, which is comprised of six serotypes. CVB1 infections cause a wide range of illnesses or symptoms of varying degrees of severity, such as hand, foot, and mouth disease (HFMD), encephalitis, myocarditis, aseptic meningitis and even death (CDC 2008; Chen et al. 2019; Ji et al. 2019; Kim et al. 2013). Outbreaks of aseptic meningitis associated with CVB1 recently occurred in eastern China (Zhang et al. 2013) and South Korea (Kim et al. 2013). In addition, CVB1, which causes significant morbidity, has constantly ranked among the top 15 enterovirus serotypes reported to the Centers for Disease Control and Prevention (CDC) in the United States (CDC 2010). Furthermore, CVB1 has also been implicated in the development of type 1 diabetes mellitus (T1DM) (Oikarinen et al. 2014; Sioofy-Khojine et al. 2018). Although CVB1 has posed a considerable public health threat, no approved vaccine or antiviral therapy is yet available.
A suitable animal model of CVB1 infection plays a critical role in pathogenesis research, vaccine evaluation and the development of antiviral reagents. The neonatal mouse model, which has the advantages of high sensitivity, good stability, low cost of obtainment and short reproductive cycle, has been widely used in the evaluation of vaccines and antiviral reagents against human enteroviruses, such as Enterovirus A71 (EV-A71) (Cao et al. 2015; Jin et al. 2017), EV-D68 (Zhang et al. 2018), Coxsackievirus A16 (CVA16) (Mao et al. 2012), CVA6 (Zhang et al. 2017), and CVA10 (Li et al. 2017) Although previous study evaluated the virulence of CVB1 mutants in newborn mice and found that they could infect and be lethal to neonatal BALB/c mice, the effects of mouse strain and age on susceptibility to infection, lethal symptoms and pathological features for CVB1 were still unclear (Zhong et al. 2008).
In this study, a neonatal mouse model was systematically established by using a clinically isolated CVB1 strain, and the pathogenic characteristics of CVB1 in mice were further analyzed. In addition, this model was also applied to evaluate the efficacy of neutralizing antibodies and candidate vaccines against CVB1.
-
Human rhabdomyosarcoma (RD) cells (ATCC, Manassas, Virginia, USA) were maintained in Dulbecco's modified Eagle medium (DMEM, 12,100-061, Gibco, Thermo Fisher, Waltham, USA) containing 10% fetal bovine serum (FBS, 10099141, Gibco). A clinical CVB1 204 strain (GenBank accession no. MT129658) isolated from a patient (Fujian, China) and a CVB1 prototype Conn-5 strain (GenBank accession no. AB061463) obtained from the American Type Culture Collection (ATCC) were used in the study. The viruses were cultured in RD cells and stored in aliquots at -80 ℃. The median tissue culture infective dose (TCID50) of CVB1 was titrated by the Reed-Muench method (Reed et al. 1938).
-
Six-week-old female BALB/c mice were immunized subcutaneously with a 0.01% formaldehyde-inactivated supernatant of the CVB1 204 strain virus emulsified in complete Freund's adjuvant and boosted twice at two-week intervals with the same antigen in incomplete Freund's adjuvant. Antisera elicited from the mice were collected at week 8, inactivated by incubation at 56 ℃ for 30 min and stored at -20 ℃ for the in vitro neutralization assay and in vivo protection assay. Then, splenocytes from immunized mice were fused with myeloma cells (Sp2/0-Ag-14), and the obtained hybridoma supernatants were screened by an in vitro neutralization assay against CVB1. Positive wells were subcloned at least twice by limiting dilution. Single-positive cells were injected into the mouse abdominal cavity to produce ascitic fluid. Monoclonal antibodies (mAbs) were purified from ascitic fluid by protein A chromatography (L00664, GenScript, Nanjing, China). The concentrations of mAbs were determined using a Bicinchoninic Acid (BCA) Protein Assay Kit (23227, Thermo Fisher). Monoclonal antibodies 5FH7 and 6H5 were screened and obtained in this experiment.
-
For comparing the sensitivity of the mouse strains for the experiments to determine the most susceptible strain, one-day-old BALB/c, C57BL/6, ICR and KM mice were challenged intraperitoneally (i.p.) with CVB1 204 strain at 103 TCID50 per mouse in a 100 μL volume. Furthermore, BALB/c mice at 1, 3, 5, 7, 14 and 21 days of age were challenged i.p. with CVB1 204 strain (103 TCID50 per mouse in a 100 μL volume) to determine the most susceptible age. In addition, the mice were challenged with ten-fold serially diluted CVB1 204 (103-100 TCID50 per mouse in a 100 μL volume) or Conn-5 (102-100 TCID50 per mouse in a 100 μL volume) strains to determine the appropriate dose. The control mice were mock-infected with an equal volume of medium via the same route. Each group contained five to eight mice. All mice were observed daily for clinical illness and death until 20 days post-infection (dpi). Clinical symptoms were scored as Table 1.
Grade Clinical sign (s) 0 Healthy 1 Lethargy and listlessness 2 Emaciation 3 Limb weakness 4 Hair thinning, hunching or weakness in ≥ two limbs 5 Moribund and death CVB1 Coxsackievirus B1. Table 1. Grading score for the clinical symptoms of CVB1-infected mice.
-
One-day-old BALB/c mice (n = 5-8) in each group were challenged i.p. with CVB1 204 strain (10 TCID50 per mouse in a 100 μL volume). When the clinical score of the mice reached ≥ 4, the mice in both of the experimental and control groups were euthanized with carbon dioxide and were subjected to histopathological and IHC examinations. The tissues other than blood were washed by sterile PBS, then the liquids were sucked dry with filter paper, and the tissues were weighed with RNA-free enzyme tubes, separately. After anaesthetization, blood samples were collected by decapitation and stored at -20 ℃. The heart, liver, spleen, lung, kidney, pancreas, intestines, brain, spinal cord, and hind-limb muscles were isolated and fixed in 4% formalin for at least three days at room temperature. Tissues were bisected, dehydrated, embedded in paraffin and were sliced into 4-mm-thick sections. Tissue sections were stained with haematoxylin and eosin (H.E.) for the histopathological examination. For the IHC examination, the tissue sections were stained using an UltraSensitive TM SP (Mouse, Rabbit) IHC and DAB Streptavidin-Biotin detection kit (KIT-9720 and DAB-0031, Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China) according to the manufacturer's protocol. The anti-CVB1 mAb 5FH7 was used as the primary antibody (1 mg/mL, 1:1000 dilution). The degree of immunostaining was scored based on the intensity index of staining. The intensity of staining was scored as follows: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). The color depth should be compared with the background color. Then, the proportion of positive cells was graded according to the following criteria: 0 (negative), 1 (positive cells ≤ 10%), 2 (11%-50% positive cells), 3 (51%-75% positive cells), and 4 (≥ 75% positive cells). The staining index was calculated as intensity score × proportion score. A staining index of > 6 was defined as strong positive, both 3 ≤ and ≤ 6 was defined as moderate positive, while < 3 was defined as weakly positive or negative.
-
To further test the dynamic changes of the various organs or tissues in one-day-old mice challenged with CVB1 204 strain (10 TCID50 per mouse in a 100 μL volume), the viral loads were measured at different time points in experimental and control BALB/c mice by RT-PCR detection (n = 3 per time point). After euthanasia, the blood, heart, liver, spleen, lung, kidney, pancreas, intestines, brain, spinal cord, and limb muscles of the CVB1-infected mice were harvested within 6, 12, 24, 48, 72, and 96 h, weighed and stored at -80 ℃. The tissues were homogenized with a high-throughput tissue homogenizer (KZ-II, Servicebio, Wuhan, China) in 500 μL PBS. Total viral RNA was extracted from tissue homogenates using a viral DNA/RNA magnetic bead method purification kit (NA007-3, GenMag Bio, Beijing, China). For quantification, CVB1 loads were performed by RT-PCR analysis with a one-step RT-PCR Kit (EZ001-2, GenMag Bio, Beijing, China) using the specific primers F-EV (5′-CTACCACTCACGGTCCGAATCGT-3′) and R-EV (5′-TGAGTTRTTRGTGTATGTGGCATAGT-3′) and a probe (5′-FAM-CCTGTGCCGGTCTGCCTGTG-BHQ-3′). RT-PCRs was carried out as follows: 15 min at 50 ℃ and 15 min at 95 ℃, followed by 40 cycles of 94 ℃ for 15 s and 55 ℃ for 45 s. The full-length CVB1 infectious cDNA clone plasmid was used as a standard sample. A standard curve, generated from serially diluted samples, was used to quantify the number of CVB1 viral copies.
-
Monolayer RD cells were diluted with DMEM and then seeded in 96-well plates at 1 × 104 cells per well. Antisera and mAb 6H5 (initial concentration of 1 mg/mL) were serially diluted twofold in DMEM ranging from 1:16 to 1:4096 dilutions, and each well was incubated in an equal volume with infectious CVB1 204 strain (100 TCID50) at 37 ℃ for 1 h. The mixtures were added to the pre-seeded RD cells and cultured at 37 ℃ for 5-7 days, and the cytopathic effects (CPEs) were observed using a microscope. The neutralizing titres were calculated based on the highest dilution that completely inhibited CPE in > 50% of the wells and taken as the average of the triplicates.
-
To evaluate the therapeutic efficacy of mAb 6H5 in vivo, the neonatal mice were challenged i.p. with CVB1 204 strain (10 TCID50 in a 100 μL volume). Then, 12 h later, a single dose of mAb 6H5 was inoculated i.p. with doses of 0.01 µg, 0.1 μg and 1 μg per body weight (g) diluted with 100 μL PBS. The mice in the control group were treated with PBS via the same route. Similarly, the therapeutic efficacy of the antisera (a dilution of 1:100) was evaluated using the same method. We monitored and recorded the mortality rates, weight and symptoms of the BALB/c mice daily until 20 days after treatment.
-
All statistical analysis was performed with GraphPad Prism software version 8.0 (GraphPad Prism Software Inc., CA, USA). The results are expressed in terms of the mean and standard deviation. Survival curves were compared by the log-rank (Mantel-Cox) test. The value of half maximal inhibitory concentration (IC50) was calculated by fitting nonlinear regression curve. A P value of < 0.05 was considered statistically significant.
Cells and Virus
Monoclonal Antibody and Antisera
Mouse Infection Experiments
Histopathological Examination and Immunohistochemical Staining (IHC)
Viral Loads in Mouse Tissues
In Vitro Neutralization Assay
Therapeutic Efficacies of the Anti-CVB1 Antibody and Sera
Statistical Analysis
-
To compare the susceptibility of different mouse strains to CVB1 infection, one-day-old BALB/c, C57BL/6, KM and ICR mice (n = 5-8) were challenged i.p. with CVB1 204 clinical strain (103 TCID50/mouse) or medium (as control groups) in a 100 μL volume. The inbred BALB/c and C57BL/6 mice died within 5 dpi, while the outbred KM and ICR mice died within 7 and 10 dpi, respectively. The results indicated that the inbred mice were more sensitive to CVB1 infection than the outbred mice (Fig. 1A). All infected mice developed weak clinical symptoms, but 100% of the mice in the control groups survived (data not shown). Compared with the other strains, BALB/c mice are an ideal experimental animal for their small individual differences, consistent genetic background and low cost, which have been widely used to establish mouse models of human enteroviruses. Therefore, we selected BALB/c mice for subsequent experiments.

Figure 1. CVB1-susceptible mouse model. A One-day-old BALB/c, C57BL/6, KM, ICR mice (n = 5-8 per group) were challenged intraperitoneally (i.p.) with 103 TCID50 per mouse of CVB1 204 strain. B BALB/c mice at 1, 3, 5, 7, 14 and 21 days of age were inoculated i.p. with CVB1 204 strain (103 TCID50 per mouse). C One-day-old BALB/c mice were inoculated i.p. with CVB1 204 strain at a dose ranging from 100 to 103 TCID50/mouse (tenfold serially diluted). D Representative pictures of mice inoculated with medium (upper portion) or CVB1 204 strain (lower portion) at 8 d post-infection (dpi), including an infected mouse exhibiting limb weakness and hair thinning (arrows), are shown. CVB1, Coxsackievirus B1; TCID50, median tissue culture infective dose.
To assess the effects of mice of various ages on susceptibility to CVB1 infection, groups of BALB/c mice at 1, 3, 5, 7, 14 and 21 days of age were challenged i.p. with CVB1 204 strain (103 TCID50/mouse). One-day-old infected mice became sick at 3 dpi, and all of them died within 5 dpi, while all of the 3-, 5- and 7-day-old infected mice died within 8, 11 and 14 dpi, respectively (Fig. 1B). In the 14-day-old infected mouse group, the survival rate was 83.4%. In contrast, all mice inoculated at 21 days exhibited no sign of disease and survived. The results indicated that the time to death or mortality of the infected mice was gradually delayed or declined with age. Therefore, one-day-old mice were the most sensitive to CVB1 infection and were selected for the following experiments.
To further determine a suitable infectious dose for the mouse model, one-day-old BALB/c mice were injected i.p. with different doses of CVB1 204 strain, ranging from 100 to 103 TCID50 (tenfold serial dilution). All mice challenged with CVB1 at 103, 102, 101 and 100 TCID50/mouse died within 14 dpi, while 100% of the mice in the control group survived (Fig. 1C). With the infectious dose of 10 TCID50/mouse, the mice began to show clinical symptoms of inactivity, emaciation, limb weakness, hair thinning and hunching at 3 dpi, and all of them died within 10 dpi (Fig. 1C, 1D). In consideration of the time to death and clinical symptoms, the challenge dose of CVB1 was chosen as 10 TCID50 per mouse.
In addition, under the above validated experimental conditions, five replicate and independent experiments were conducted at different times to evaluate the repeatability and stability of the established mouse model of CVB1. As shown in Table 2, in these five experiments, mice began to become sick between 3 and 4 dpi and died between 4 and 5 dpi, and 100% mortality rates occurred between 8 and 11 dpi. The results indicated that the established experimental conditions of the CVB1 mouse model presented good reproducibility.
Experiment Number of mice Onset of symptoms (d) Onset of death (d) The time of all mice died (d) Mortality ratio (%) 1 7 3 4 10 100 2 6 3 4 10 100 3 6 3 4 8 100 4 8 4 5 11 100 5 6 3 4 9 100 The results of five replicate and independent experiments that were challenged by a CVB1 clinical stain (10 TCID50 per mouse) in neonatal BALB/c mice at different times are shown. The clinical symptoms and mortality were monitored and recorded daily, which showed good reproducibility.
CVB1 Coxsackievirus B1; TCID50 median tissue culture infective dose.Table 2. The repeatability and stability of the CVB1-challenged neonatal mouse model.
To further explore the universality of the mouse model, one-day-old mice were challenged i.p. with different doses of CVB1 Conn-5 prototype strain. Similarly, the results showed that there was a dose-response effect between the infectious dose of virus and the mortality rate of mice, and 100% of mice challenged with Conn-5 strain at 102 TCID50/mouse died within 5 dpi (Supplementary Fig. S1).
-
To investigate the pathological changes, various tissues derived from CVB1-infected mice (grade level 4) and medium-injected mice (control) were used for histopathological examinations. H.E. staining of tissues from infected mice showed that the pancreas exhibited severe cellular necrosis with inflammatory infiltration compared with the normal tissue (Fig. 2A, 2C). IHC results confirmed that a massive spread of CVB1 antigen was present in the pancreas from infected mice with a staining index more than 3, but absent in the control mice (Fig. 2B, 2D). In addition, moderate positive signals for CVB1 were detected in the heart, spinal cord (the anterior horn cells), limb muscle and kidney without pathological damage (Fig. 2E-2L). However, no histological changes or viral antigens were observed in the other detected tissues of the infected mice, such as the brain (including the brain stem), liver, intestine and lung (data not shown). These results indicated that CVB1 infection caused pathological damage in the neonatal mice, especially damage to pancreatic cells.
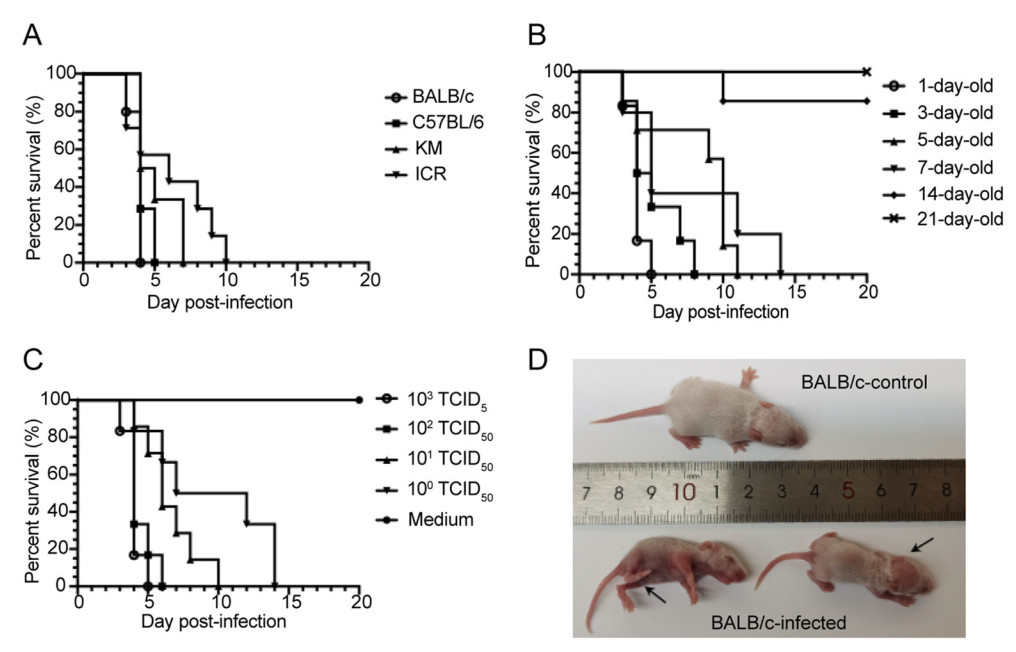
Figure 2. Histopathological examination of tissues from mice infected with lethal doses of CVB1. When the clinical score of infected mice reached ≥ 4 (approximately 7 dpi), the mice were euthanized and subjected to H.E. staining (A, C, E, G, I and K) and immunohistochemistry (IHC) examinations (B, D, F, H, J and L). Bar = 50 μm. The experiments were repeated independently in triplicate and one representative result was shown. H.E., haematoxylin and eosin; IHC, immunohistochemistry; CVB1, Coxsackievirus B1; dpi, day post-infection.
-
To further understand the dynamics of viral loading in mice challenged with CVB1, blood and different tissue samples of the heart, liver, spleen, lung, kidney, pancreas, intestines, brain, spinal cord and limb muscles were analyzed by RT-PCR at different time points post-infection. As shown in Fig. 3, CVB1 was hardly detected in all collected tissues and blood at 6 h post-infection (hpi), but viruses could be detected in most tissues and blood at relatively low viral copies (5-104 copies/mg or /mL) at 48 hpi. With time, viral copies of CVB1 continued to increase in most tissues and blood, except for a decrease in liver, kidney and limb muscle at 72 hpi, which demonstrated that all tissues could be infected with CVB1 by the middle stage of infection. By comparison, the viral load in the liver increased more rapidly to a level of 3.89 × 104 copies/mg in the first 48 hpi, which indicated that the liver might be the target organ of CVB1 infection at the early stage. However, the viral loads in the blood and pancreatic tissue increased most rapidly throughout the experiment, reaching 2.13 × 108 copies/mL and 7.72 × 108 copies/mg, respectively. These results suggested that mice infected with CVB1 might exhibit viraemia and that CVB1 had a strong tropism for pancreatic tissue in neonatal mice, which was consistent with the H.E. and IHC results.
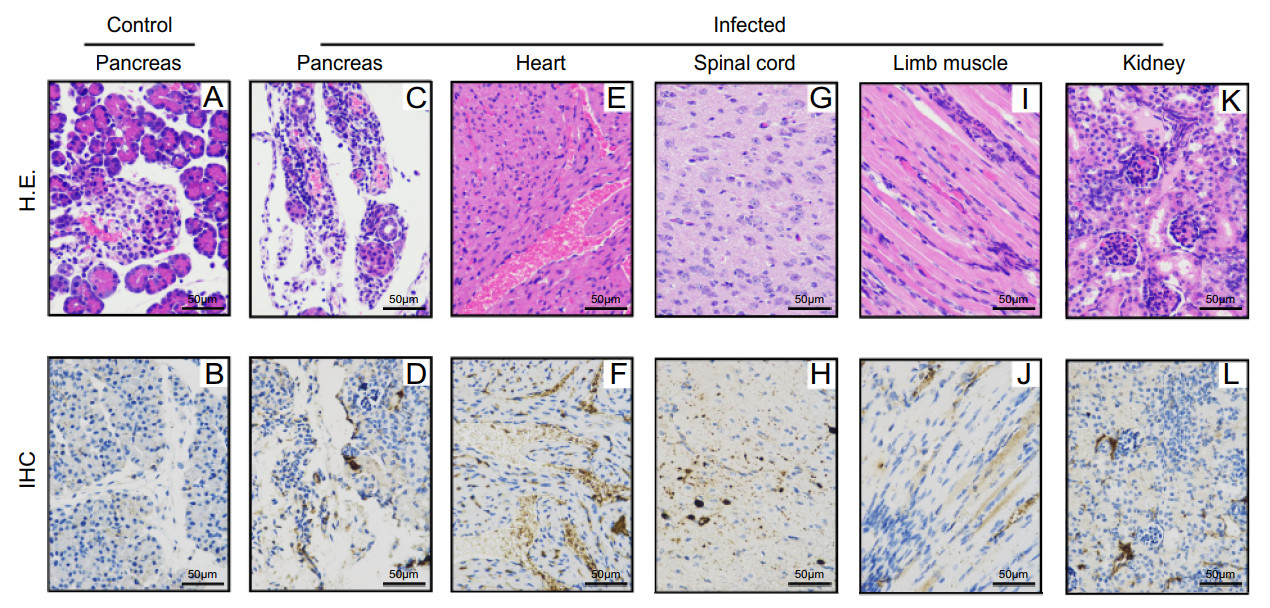
Figure 3. Tissue viral loads in CVB1-infected mice at different times. One-day-old mice were inoculated i.p. with 100 μL of CVB1 (10 TCID50 per mouse). Blood and tissue samples of heart, liver, spleen, lung, kidney, pancreas, intestine, brain, spinal cord and limb muscle were collected at 6, 12, 24, 48, 72 and 96 h post-infection (n = 3, per time point). Viral loads were measured by RT-PCR, and the results showed the mean ± SEM of the viral load (n = 3). Mice in the control group did not show any significant viral loads which were not shown. SEM, standard error of mean; TCID50, median tissue culture infective dose; CVB1, Coxsackievirus B1; i.p., intraperitoneally.
-
A mAb against CVB1, named 6H5, was produced in our laboratory and exhibited a high neutralizing activity in vitro (IC50 = 0.318 μg/mL) (Fig. 4A). The in vivo therapeutic efficacy of mAb 6H5 was evaluated in the established neonatal mice. Different doses of mAb 6H5 were injected via the i.p. route 12 h after the mice were challenged with a lethal dose of CVB1 (10 TCID50 per mouse). As shown in Fig. 4B-4D, all of the mice treated with mAb 6H5 at a concentration of 0.1 μg/g or 1 μg/g survived and were healthy throughout the experiment, while the mice treated with 0.01 μg/g mAb 6H5, similar to the mice in the control group, started to show signs of illness at 3 dpi and all died within 8 dpi. The results showed that the neutralizing antibody 6H5 had a 100% protective effect against a lethal challenge with CVB1 at concentrations higher than 0.1 μg/g. To further evaluate the efficacy of 6H5, mice were infected i.p. with a dose of 0.1 μg/g at 24, 48 or 72 hpi. The results showed that all mice received 6H5 at 24 hpi were totally survived and healthy, while treatment at 48 or 72 hpi could not protect mice from death respectively, indicated 6H5 might be suitable for the early treatment of CVB1 infection (Supplementary Fig. S2).
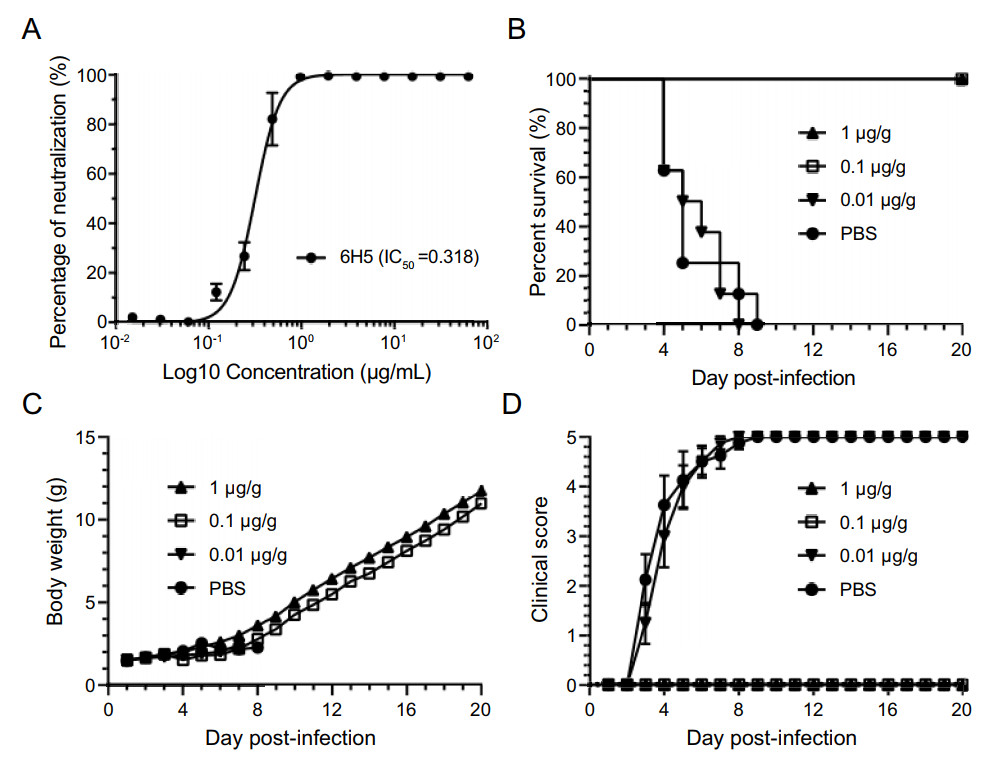
Figure 4. The application of the CVB1 neonatal model in the evaluation of neutralizing antibody drugs. A Neutralization efficiency of the mouse neutralizing antibody 6H5 against CVB1 204 stain (IC50 is marked). Data were shown as mean ± SD. B-D To evaluate the therapeutic effect of mAb 6H5 in this model, the neonatal mice were challenged with lethal doses of CVB1 (10 TCID50 per mouse). At 12 h post-infection, the therapeutic mAb 6H5 was inoculated i.p. at concentrations of 0.01 μg, 0.1 μg and 1 μg per body weight (g) in 100 μL PBS (n = 5-8 per group). The control group was injected with the same volume of PBS only. The mortality (B), body weight (C), and clinical symptoms (D) of all mice were monitored daily until 20 dpi. Data were shown as mean ± SEM in (C) and (D). CVB1, Coxsackievirus B1; TCID50, median tissue culture infective dose; IC50, half maximal inhibitory concentration; i.p., intraperitoneally; mAb, monoclonal antibody; dpi, days post-infection.
-
Three antisera elicited from CVB1-vaccinated mice showed high neutralizing titres against CVB1 (Fig. 5A). The in vivo therapeutic efficacy of these antisera was evaluated using the established neonatal model of CVB1. Mice were injected i.p. with anti-CVB1 sera (a dilution of 1:100) after inoculation with CVB1 as previously described. The results showed that the mice in the control group started to become sick at 4 dpi and that 100% of mice died within 11 dpi. In contrast, the mice treated with anti-CVB1 sera all survived without showing any significant clinical symptoms throughout the experiment (Fig. 5B-5D).
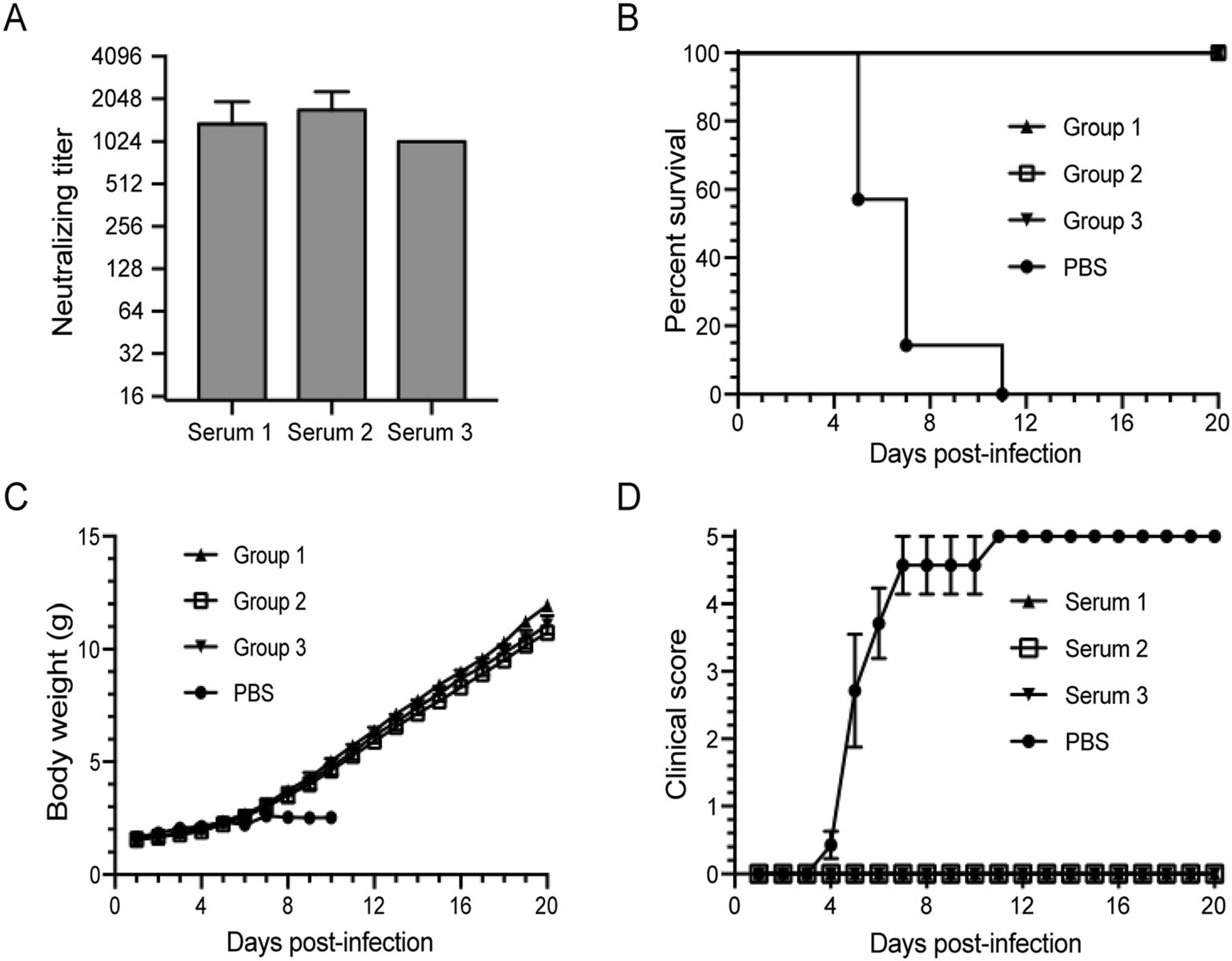
Figure 5. The application of the CVB1 neonatal model in the evaluation of the inactivated CVB1 vaccine. Three antisera were diluted 100-fold and were used to treat the neonatal mice that were challenged i.p. with CVB1 204 stain (10 TCID50 per mouse) in groups 1, 2 and 3, respectively. A Neutralization titres of the antisera elicited from CVB1-vaccinated mice. Data were shown as mean ± SD. B-D The experimental group mice were challenged i.p. with 10 TCID50 per mouse of CVB1 204 stain. At 12 h post-infection, the antisera were inoculated at a dilution of 1:100 in 100 μL PBS per mouse (n = 5-8, per group). Group 1 was treated with serum 1, group 2 was treated with serum 2, group 3 was treated with serum 3, and the PBS control group was injected with the same volume of PBS. The mortality (B), body weight (C), and clinical symptoms (D) of all mice were monitored daily until 20 dpi. Data were shown as mean ± SEM in (C) and (D). CVB1, Coxsackievirus B1; TCID50, median tissue culture infective dose; i.p., intraperitoneally; dpi, days post-infection; SD, standard deviation; SEM, standard error of mean.
-
CVB1 has emerged globally as a predominant pathogen of severe human infectious diseases and has posed a substantial public health threat. In recent years, scientists have started trials on the potential prevention and treatment options for CVB1 infection (Hankaniemi et al. 2019a, 2019b; Heinimäki et al. 2019; Hober and Alidjinou 2018; Larsson et al. 2015; Zeng et al. 2020), which has increased the demand for animal models of CVB1. Previously, Larsson et al. (Larsson et al. 2015) and Hankaniemi et al. (Hankaniemi et al. 2017) assessed the immunogenicity of an inactivated CVB1 vaccine using BALB/c and nonobese diabetic (NOD) mice, and Stone et al. (Stone et al. 2018) further verified its safety and efficacy using SOCS1-tg mice. These murine models used mice that were between 4 and 7 weeks of age and were mainly used to assess the ability of the CVB1 vaccine to protect against virus-induced diabetes. Given the diversity and severity of the diseases caused by CVB1 infections, it is necessary to establish other animal models for studying the characteristics of CVB1 infections more comprehensively and to evaluate the protective effects of vaccines and antiviral reagents more rapidly and efficiently, such as neonatal mouse models. However, a neonatal mouse model of CVB1 has not been systematically developed. In this study, a lethal model of CVB1 in newborn mice was successfully established based on a clinical strain isolated from a meningitis patient in Fujian Province in 2014. We found that CVB1 infection could cause 100% mortality of several mouse strains, but the survival rate of BALB/c mice increased with age or decreased with increasing infection dosage. The results suggested that the age of the mice and the dosage of virus infection, rather than the mouse strain were the main limiting factors for efficient CVB1 infection in neonatal mice.
CVB might target islet β cells to initiate inflammation and autoimmunity, resulting in β cell damage, which triggered the development of T1DM (Hober and Alidjinou 2018; Op de Beeck and Eizirik 2016; Stene et al. 2010). Tracy et al. assayed nine CVB3 strains in C3H/HeJ (H2k) mice and found that eight of nine CVB3 strains could readily induce acute pancreatitis in mice (Tracy et al. 2000). Yanagawa et al. confirmed that early CAR-Fc treatment could completely block pancreatic inflammation and cell death in A/J mice (Yanagawa et al. 2004). Pancreatic affinity can also be found in CBA/J mice infected with CVB4 (Hindersson et al. 2004). In previous study, NOD and SOCS1-tg mice were selected and inoculated with CVB1. Viraemia and viral replication in the pancreas were measured using standard plaque assay and PCR. Moreover, pancreas showed signs of pancreatitis and was positive for viral protein in CVB1-infected mice, indicating that CVB1 exhibited strong tropism to pancreas tissue (Stone et al. 2020, 2018). Similarly, the current study showed that CVB1 infection had a strong tropism towards pancreatic tissue in neonatal mice, causing massive inflammatory lymphocyte infiltration, significant changes in tissue morphology, and acinar tissue cytolysis. Moreover, the viral loads in the pancreas of CVB1-infected mice increased rapidly and significantly. Taken together, these findings may provide some support for the close association between CVB1 and pancreatitis or T1DM.
CVB1 infection also caused viral myocarditis and meningitis clinically (Callen and Paes 2007; Goren et al. 1989; Lu et al. 2005). In this study, pathological examination and tissue viral load results showed a positive signal in the heart and spinal cord in moribund mice. Moreover, high copy number of virus could be detected in brain after CVB1 challenge, and the mice also showed clinical symptoms of inactivity and dull. These results suggested that CVB1 could infect the heart and brain of neonatal mice, which could inform the clinical cases of meningitis and myocarditis in neonatal infant following CVB1 infection (CDC 2008). In addition, a higher amount of CVB1 RNA was detected in the blood throughout the experiment, suggesting that CVB1 could enter into the blood circulation by mesenteric infection and then widely spread to various tissues for replication and distribution. Viraemia spread may be one of the causes of death in CVB1-infected mice.
Neutralizing antibodies are considered to be effective and necessary for protection against virus infection. Passive immunization with CVB1-specific neutralizing antibodies may provide protection for CVB1-susceptible patients with severe clinical symptoms (Wikswo et al. 2009). In this study, we evaluated the therapeutic potential of a CVB1 neutralizing mAb 6H5, produced in our laboratory, using the established neonatal mouse model. Passive immunization with mAb 6H5 at a dose of 0.1 μg/g had a 100% protective effect against lethal CVB1 challenge, suggesting that 6H5 was a potential therapeutic mAb for further humanization. Similarly, the antisera from CVB1-vaccinated mice could also effectively protect 100% of neonatal mice from the lethal challenge of CVB1 in our mouse model. It should be noted that the incubation period and the death are short few days away from infection in one-day-old mouse model. Therefore, the 3- to 7-day-old mice could also be used to evaluate the protective effects of CVB1 vaccines and antiviral reagents in the further research, since there was also consistent mortality.
In conclusion, we successfully established a lethal neonatal mouse model using a clinical CVB1 isolate strain and demonstrated that CVB1 had strong tropism to the pancreas, resulting in pancreatic cell damage. Importantly, our mouse model could be effectively used to preliminarily evaluate the efficacy of antiviral reagents and vaccines against CVB1.
Establishment of a CVB1-Susceptible Neonatal Mouse Model
Pathological Changes in CVB1-Infected Mice
Tissue Viral Loads in CVB1-Infected Mice
Evaluation of the Neutralizing Antibody 6H5 in the Mouse Model
Evaluation of Anti-CVB1 Sera in the Mouse Model
Discussion
-
This research was supported by grants from the National Natural Science Foundation of China (No. 82072282 and 81801646), the National Science and Technology Major Project of Infectious Diseases (No. 2017ZX10304402-002-003) and the National Science and Technology Major Projects for Major New Drugs Innovation and Development (No. 2018ZX09711003-005-003). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
-
NX, TC and YW conceived the experiments. ZY, RZ and LX wrote the manuscript. ZY and YW performed the majority of the laboratory work. YL and HY prepared of plasmids. WF, QH and DZ checked and finalized the manuscript. JW and WW prepared Coxsackievirus B1 for this study. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interests.
-
All of the animal experiments in this study were approved by the Institutional Animal Care and Use Committee at Xiamen University and conducted in accordance with animal ethics guidelines and approved protocols.
















 DownLoad:
DownLoad: