-
CD4+CD25+ regulatory T cells (Tregs) have been demonstrated to play a crucial role in mediating immunotolerance and suppressing the activation and proliferation of innate and adaptive immunocytes (21-23). It has also been implicated in controlling excessive immune responses to chronic pathogens and in limiting immunopathology (4). In human, the T cell subset is identified by high expression of IL-2Rα chain (CD25) and the forkhead/winged helix trans-cription factor (FoxP3), which has been demonstrated to be a unique mark restricted to the T regulatory cells (3, 19).
Human immunodeficiency virus type-1 (HIV-1) in-fection is characterized by a progressive loss of CD4+ T cells and a wide array of immune dysfunc-tions, including chronic immune activation and paradoxical anergy of immunocytes (6, 14). Emerging evidence support the hypothesis that pathogenesis of virus chronic infection may be directly related to the levels of circulating CD4+CD25+ Treg or to the balance of the Treg versus effector T cells. In hepatitis C virus (HCV) and hepatitis B virus (HBV) infected subjects, CD4+CD25+ Treg may contribute to the persist infections by down-regulating antigen-specific T cell response (5, 25). But in HIV infection, how Treg regulates immune responses and correlates with disease progression in chronic HIV infection remains unknown (2, 8, 12, 16, 24). A recent study suggested that Tregs are generally depleted in HIV infection and their loss may facilitate the immune hyperactivation (7). But some other studies supported the notion that Tregs contribute to HIV-specific immune dysfunction by limiting immunoreactions (10, 24). However, whether circulating Treg was increased or not in HIV infection is not very clear.
In this study, we hypothesized that functional Tregs might mediate the immune dysregulation in chronic HIV infection. It was found that with the disease progression, the circulating Treg frequency was significantly increased; while Treg absolute counts were decreased. And there was a negative correlation between circulating Treg frequency and CD4 counts. More important, our data indicated Treg could inhibit both CD4+ and CD8+ T cell proliferation in vitro, and then might result in decreasing CD4 and CD8 T cell counts. These findings suggested that the alteration of Tregs may act as an important prognostic marker for the disease progression of chronic HIV infection.
HTML
-
A total of 75 HIV-1 infected antiretroviral-na ve individuals were enrolled in this study. HIV seroposi-tivity was determinated by enzyme-linked immuno sorbent assay (ELISA) and confirmed by Western blot analysis. All these individuals were paid blood donors and infected during the period of 1994-1995. No evi-dence of active opportunistic infections and tumors were found for all of HIV-infected subjects at the time of blood sampling. Further exclusion criteria included pregnancy, active tuberculosis (TB; defined as sus-pected TB or in the first 2 mo of anti-TB therapy), or moribund status (7). Thirty healthy subjects with age and gender-matched were employed as normal con-trols (NC). The basic characteristics of these individuals were shown in Table 1. The study protocol was approved by the Ethics Committee of our unit, and written informed consent was obtained from each subject.

Table 1. Characteristics of subjects in the study
-
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifu-gation from heparinised blood sample. CD4+CD25+ Treg were isolated from PBMC by CD4 negative selection followed by CD25 positive selection, using CD4+CD25+ T-cell isolation kit (Miltenyi Biotech, Bergisch-Gladbach, Germany) according to the manu-facturer's instructions. Treg-removed PBMCs were collected for next experiments. Treg frequency in Treg-removed PBMCs was < 0.5%, as determined by flow cytometric analysis.
-
All antibodies were purchased from BD Phar-Mingen (San Diego, USA), except for anti-FoxP3 antibody from eBiosciences (San Diego, USA). For staining of Treg, the cells were first stained with PerCP-anti-CD3, APC-anti-CD4 and PE-anti-CD25 Abs, then permeabilized and fixed using eBioscience fix/perm (eBiosciences, San Diego, USA) according to the manufacturer's instructions. After 30-minute permeabilization, FITC-anti-FoxP3 was added for another 30 minutes. Four-color flow cytometric analysis was performed using FACSCalibur and CELLQuest software (Becton Dickinson, San Jose, USA).
-
The proliferation of PBMCs was analyzed via CFSE [5-(and 6-)carboxyl-fluorescein diacetate, suc-cinimidyl ester] labeling assay as described previously (11, 27). In brief, PBMCs and Treg-removed PBMCs were incubated in PBS containing 0.1% BSA with 5 μmol/L CFSE for 10 min at 37 ℃. Then labeling was quenched with RPMI1640 containing 10% FCS on ice for 5 min and cells were washed twice with PBS. The CFSE-labeled cells were seeded at 5×105 cells/mL in 96-well plates. PBMCs were stimulated with anti-CD3 and anti-CD28 (1 μg/mL) for 96 h in vitro. Proli-feration was analyzed using FACSCalibur (Becton Dickinson, San Jose, USA).
-
The 7900HT Sequence Detection System (Applied Biosystems) was used to quantify HIV-1 RNA levels in plasma samples in our laboratory. The cut-off value was 500 copies/mL. The protocol was previously described (13).
-
Data were analyzed using SPSS 13.0 for Windows software (SPSS Inc., Chicago, USA) and expressed as mean and standard deviation. Multiple comparisons Kruskal-Wallis H nonparametric test was applied with Bonferroni step down (Holm) correction. Mann-Whi-tney nonparametric U test was used for difference between two groups. Wilcoxon signed ranks test was used for two-related-samples test. Spearman cor-relation analysis was performed between two para-meters. P < 0.05 is considered as a significant diffe-rence.
Subjects
Cell isolation and sorting
Flow cytometric analysis
CFSE proliferation assay
Plasma HIV-1 RNA monitoring
Statistical analysis
-
Tregs are defined as CD3+CD4+CD25+FoxP3+ T cell population. There was a significantly higher fre-quency of circulating Tregs in HIV-1 infected patients than in health controls (mean 8.25 ± 3.49% vs 4.52 ± 1.31%, P < 0.001) (Fig. 1a). On the contrary, Treg absolute counts were significantly reduced in HIV-1 infected patients compared with health controls (19 ± 9/μL vs. 31 ± 9/μL, P < 0.001).
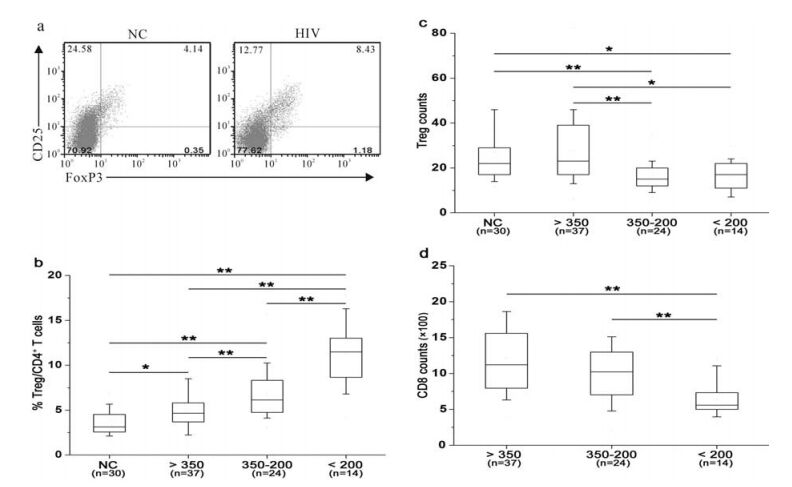
Figure 1. HIV-1 infected patients displayed an increased Treg frequency and reduced absolute counts with disease progression. (a) Representative prevalence of CD4+ CD25+ FoxP3+ Treg from a health individual and an HIV-1 infected patient. The values in right upper quadrant indicate the percentage of CD4+CD25+FoxP3+ cells in CD4 T cells. (b) Comparisons of Treg frequency in the four groups. (c) Comparisons of Treg absolute counts in these four groups. (d) Comparisons of CD8 T cell counts in HIV-positive groups. Mann-Whitney nonparametric U test were used for comparison between two groups. Data in b, c and d were expressed as box plots in which the horizontal lines illustrated the 25th, 50th, and 75th percentiles. Vertical lines represented the 10th and 90th percentiles.
-
To investigate the correlation between Tregs and disease progression, all HIV-infected patients were divided into four groups according to CD4 counts. Health individuals were enrolled in Group A. Patients with mild immunodeficiency stage (CD4≥350 cells/ μL) were in Group B; while patients with advanced immunodeficiency stage (200≤CD4 < 350 cells/μL) were in group C. Patients in Group D displayed a severe immunodeficiency stage (CD4 < 200cells/μL). It was found that Treg frequency was gradually increased with the disease progression (Fig. 1b); while Treg absolute counts were gradually decreased with the disease progression (Fig. 1c). In addition, circu-lating CD8 T cell counts gradually decreased with the reduction of CD4 T-cell counts (Fig. 1d). These data show that circulating Treg frequency gradually dec-reased along with the disease progression indicated by CD4 T-cell absolute counts; while Treg number was simultaneously increased.
Correlation between Treg frequency or absolute counts and circulating CD4 T-cell absolute counts was further analyzed in this study. There was a significant negative correlation between Treg frequency and circulating CD4 T-cell absolute counts in these HIV-infected patients (r = -0.512, P < 0.001, Fig. 2a). However, the Treg counts were positively correlated with CD4 counts (r = 0.503, P < 0.001, Fig. 2b). In addition, there was a significant positive correlation of Treg counts with circulating CD4 T-cell absolute counts in these HIV-infected patients ((r = 0.291, P= 0.011, Fig. 2d); while no significant correlation was found between Treg frequency and CD4 T-cell absolute counts (Fig. 2c). These data suggest that Treg frequency and counts significantly correlate with disease progression indicated by CD4 T-cell number in HIV infection.
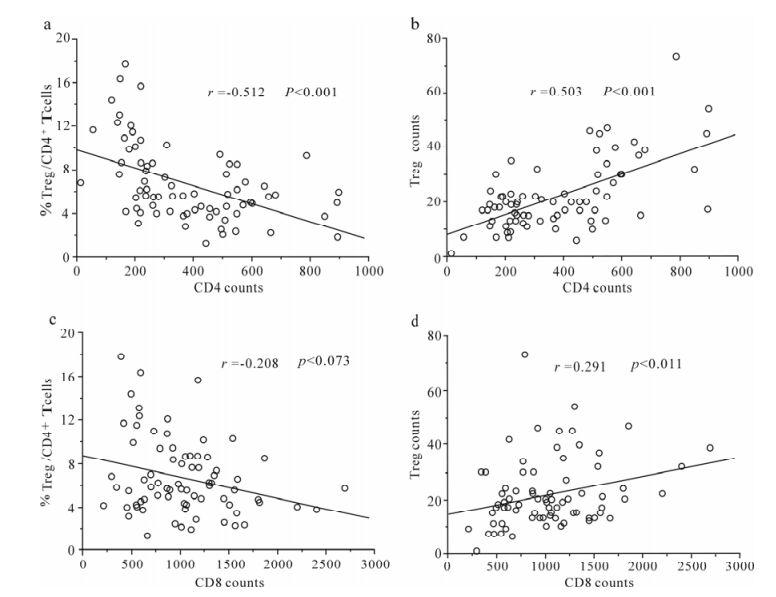
Figure 2. Correlation analysis between Treg frequency or counts changes and CD4 or CD8 counts. (a) Treg frequency was reversely correlated with CD4 counts in HIV infected patients. (b) Treg absolute counts were positively correlated with CD4 counts in HIV infected patients. (c) No significant correlation was found between Treg frequency and CD8 counts. (d) Treg absolute counts were positively correlated with CD8 counts in HIV infected patients. Spearman correlation analysis was performed. Solid line, linear growth trend; r, correlative coefficient.
-
To observe effect of Treg on T-cell proliferation, we compared the proliferative capacity of PBMCs and Treg-removed PBMCs stimulated by anti-CD3 and anti-CD28 antibodies using CFSE label assay (Fig. 3). We observed that TCR stimulation could induce both CD4 and CD8 T cells of HIV-infected patients to proli-ferate; while deletion of Treg from PBMCs could significantly enhance proliferative capacity of both CD4 and CD8 T cells (Fig. 3). These data showed that Tregs suppress both CD4 + and CD8+ T cell proli-feration in vitro.
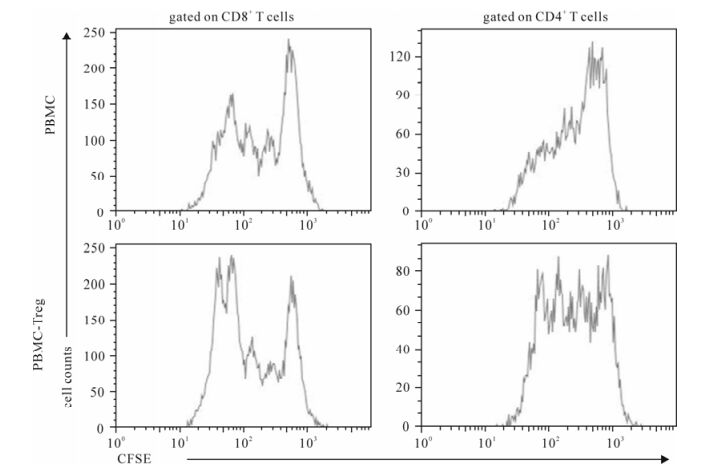
Figure 3. Deletion of Treg from PBMC significantly enhanced proliferation of both CD4+ and CD8+ T cells. Both PBMCs and PBMC-Treg were stimulated by anti-CD3 and anti-CD28 antibodies for 96 hours. CFSE labeling assay was performed. The representative histogram showed that both CD4 and CD8 T cells from PBMCs-Treg have higher level of proliferation as compared with those from PBMCs in HIV infection.
Increased Treg frequency and reduced counts in HIV-1 infection
Treg frequency and absolute number correlate with disease progression of HIV-1 infection
Treg suppress proliferation of CD4 and CD8 T cells in vitro
-
In this study, we enumerated the Treg absolute counts and frequency in antiretroviral-na ve HIV-infected patients. Compared with normal control, HIV-infected patients displayed a significant decline in Treg absolute counts but a significant increase in Treg frequency. In addition, our finding has demon-strated that Tregs could efficiently inhibit both CD4 and CD8 T-cell proliferation in vitro. So Treg depletion might play a critical role in pathogenesis of chronic HIV infection
In general, CD4 counts were considered as a marker of HIV disease progression. In this study, it was found that Treg absolute counts positively correlated with CD4 counts, suggesting Treg absolute counts might also severe as a marker of HIV disease progression. These data also indicate that Tregs could also be depleted in HIV infection. However, our data show that Treg frequency reversely correlated with CD4 counts, suggesting Treg might have a slower decline compared with other CD4 T cells and displayed a distinct kinetics from other CD4 T cells in chronic HIV infection. Persistent pathogens which have been reported to promote the expansion and activation of antigen-specific CD25+ CD4+ Treg cells, and to induce normal CD4+ T cells to gain CD25+ T cell phenotype and function (9, 26), might contribute to a slower decline of Tregs, although we could not exclude other factors such as differential apoptosis induced by overactivation and various degree of killing effect of virus between Tregs and other CD4 T cells.
Several studies have shown that Treg could efficiently suppress cytokine production and proli-ferative capacity of CD8+ T cells in HIV infection (1, 20, 24). Our data suggest that Treg can suppress both CD4 and CD8 T cell proliferation in vitro, which also supported the previous opinion (1, 20, 24). The inhibitory effect of Tregs on CD4 and CD8 T cell proliferation might partly explain the decline of peripheral CD4 and CD8 T cell counts with the disease progression. This conclude is based on the evidence that there are significant negative corre-lations between Treg frequency and peripheral CD4 and CD8 T cell numbers. Interestingly, our data showed that Treg absolute counts positively correlate with circulating CD4 or CD8 T-cell counts. These data indicate Treg might have positive effects on CD4 and CD8 T-cell counts in vivo and depletion of Treg might improve the disease status of HIV infection. The study from Oswald-Richter group also supported the notion that Tregs play a beneficial role as low Foxp3 mRNA levels in the CD25brightCD4+ T cell population is associated with elevated CD4+ T cell activation (18). Kinter et al reported that CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulated CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status (10). Thus, Tregs may play a crucial role through mediating a inhibition of hyperimmune activation in these patients (17, 20). Future studies needs to confirm the function of Treg in chronic HIV infection.
Taken together, our data showed that Treg counts were closely correlated with disease progression in chronic HIV infection, suggesting that Treg absolute counts might also severe as a marker for disease progression. Our findings indicates that Treg actively participate in pathogenesis of chronic HIV infection.













 DownLoad:
DownLoad: