-
Classical swine fever virus (CSFV), in the Pestivirus genus and the Flaviviridae family, is a small, enveloped, positive and single-stranded RNA virus. CSFV is the causative agent of Classical swine fever (CSF). CSF, also known as 'hog cholera', is a highly contagious febrile disease worldwide. Outbreaks of CSF cause heavy losses in pig production and severely hamper the international trade in livestock. For these reasons, CSF is listed as a notifiable disease by the Office International des Epizooties and the European Union[1, 9]. Thus early diagnosis of the disease is important to effectively control the spread of the CSFV.
Virus isolation is the 'gold standard' for CSFV diagnosis. However, it cannot meet the rapid detection requirements because it is labor intensive and time consuming. Direct fluorescent antibody test of frozen sections is rapid and reliable, but needs to performed by well-trained technicians. Antigen-capture enzyme-linked immunosorbent assays are suitable for early diagnosis but are less sensitive. Recent molecular techniques based on genomic sequence detection such as reverse transcription PCR (RT-PCR), reverse transcription nest PCR (RT-nPCR) and real-time PCR are sensitive and rapid, but these methods use thermocycling equipment and so are impractical for field applications and for use in less developed laboratories [5].
Recently, a novel, rapid and sensitive technique named loop-mediated isothermal amplification (LAMP) was developed by Notomi et al[8]. It can amplify a target gene (wither a DNA or an RNA template) in a very short time with high sensitivity, specificity and efficiency under isothermal conditions [6, 7, 10, 13]. For these reasons, LAMP has been wildly used for virus diagnosis [2, 3, 11, 14]. In this study, a one-step, single-tube RT-LAMP assay was developed for the rapid detection of CSFV.
HTML
-
The reference strains CSFV SM, HCLV and Thiverval strains were provided by the China Veterinary Culture Collection Center. The other CSFV strains BJ1/08, BJ2/08, JL1/94(blood), GXBH1/98, HeBZJK1/99, BJCY1/96, USA-331, JL1/94(cell culture), GDGZ1/95 and FJFQ1/98 were isolated and maintained by the National Classical Swine Fever Reference Laboratory, China. Bovine viral diarrhea virus (BVDV), Porcine circovirus (PCV), Porcine reproductive and Respiratory syndrome virus (PRRSV), Swine influenza virus (SIV), Porcine parvovirus (PPV) and Pseudorabies virus (PRV) were also obtained from the China Veterinary Culture Collection Center.
-
A total of 157 acute-phase samples, including blood and tonsil, collected from dead pigs with a clinical diagnosis of Classical swine fever and admitted to the National Classical Swine Fever Reference Laboratory, China, were used to evaluate the RT-LAMP method by comparison with RT-PCR. All the samples were transported to the laboratory under strict cold chain conditions and stored at -80℃ until further investigation.
-
Total RNA was extracted with Trizol reagent (Invitrogen, USA) according to the manufacture's instructions. After elution with 20 μL nuclease-free double-distilled H2O, RNA samples were stored at -70℃ until required. Total DNA was extracted using the TIANamp Genomic DNA Kit (Tiangen Biotech, Beijing, China) according to the manufacture's instructions. After extraction, DNA was eluted with 20 μL elution buffer and stored at -20℃ for analysis.
-
To find conserved regions of the CSFV genome, 28 genome sequences of CSFV (GenBank accession numbers: A16790, AF091661, AF092448, AF326963, AF333000, AF352565, AF407339, AY072924, AY367767, AY554397, AY568569, AY578687, AY578688, AY646427, AY663656, AY775178, AY805221, D49532, D49533, DQ127910, J04358, L49347, M31768, NC_002657, U45478, U90951, X87939, X96550) were downloaded from GenBank and aligned using the Vector NTI 10 program. Several sets of six primers (two outer, two inner and two loop primers) were designed with the Primer Explorer V4 software (https://primerexplorer.jp) according to these potential target regions and one set of primers was selected based on the criteria described by Notomi et al (Fig. 1)[8].
-
In order to test the sensitivity of the RT-LAMP assay, RT-PCR was performed using a CSFV RT-PCR kit (Anheal Laboratories, Beijing, China). The same RNA templates were used for both RT-LAMP and RT-PCR. Positive and negative controls were used for each reaction and all reactions were carefully monitored for cross-contamination.
-
The RT-LAMP reaction was carried out in a total volume of 25 μL, with mixture of 1×Thermo buffer (New England Biolabs, USA), 0.8 mol/L Betaine (Promega, USA), 1.4 mmol/L dNTP mix (Dingguo, China), 4mmol/L MgSO4(Promega, USA), 8 U Bst DNA polymerase (New England Biolabs, USA), 2 U AMV reverse transcriptase (Promega, USA), 5 pmol/L F3 and 5 pmol/L B3, 50 pmol/L FIP and 50 pmol/L BIP, 25 pmol/L LF and 25 pmol/L LB and 2 μL of template RNA. The mixture was incubated at 63 ℃ for 60 min, and then heated at 80 ℃ for 10 min to stop the reaction. RT-LAMP assay products (10 μL) were electrophoresed on a 2% agarose gel in Tris-borate buffer. The result was visualized with a UV transilluminator at 302 nm. The amplification should be seen as a ladder-like pattern on the gel due to the formation of a mixture of stem-loop DNAs with various stem lengths and cauliflower-like structures, with multiple loops induced by annealing between alternately inverted repeats of the target sequence in the same strand.
To facilitate field application of the RT-LAMP assay, we also monitored the reaction visually. A positive amplification was seen as either the precipitation of the white by-product magnesium pyrophosphate in the tube bottom after a pulse spin [13] or by a color change following the addition of 1 μL of SYBR Green I dye (Invitrogen, USA) to the tube.
-
To confirm the specificity of the RT-LAMP assay, cross-reactivity tests were conducted using different sources of RNA or DNA as templates. Restriction endonuclease digestion was used simultaneously to further confirm the specificity.
Viruses isolates
Clinical samples
RNA and DNA extractions
Design of CSFV-specific RT-LAMP primers
RT-PCR
RT-LAMP assay
Specificity tests
-
The sensitivity of the CSFV RT-LAMP assay was determined using 10-fold serial dilutions of RNA extracted from 0.2 mL of 103.84TCID50blood samples containing the SM virus. The results showed that the accelerated RT-LAMP assay was 100-fold more sensitive compared with conventional RT-PCR, the detection limit of RT-LAMP being a 10-6 dilution of template RNA (Fig. 2).
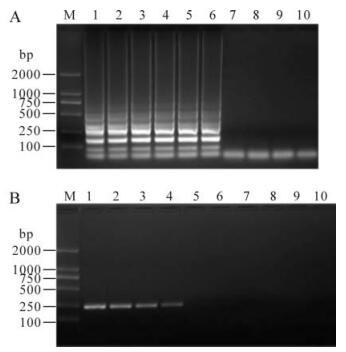
Figure 2. Comparative sensitivity of RT-LAMP and RT-PCR. RT-LAMP products(A) and RT-PCR products(B). M, 2000-bp DNA marker; 1-8, RT-LAMP products or RT-PCR products of dilution RNAs extracted from the 200μL blood stock of CSFV-Shimen (103.84 TCID50/mL). 1 lane, Template concentration 10-1; 2 lane, 10-2; 3 lane, 10-3; 4 lane, 10-4; 5 lane, 10-5; 6 lane, 10-6; 7 lane, 10-7; 8 lane, 10-8.
-
The RT-LAMP assay detected 13 different isolates of CSFV, each showing the characteristic ladder-like pattern in the gel, the formation of a white precipitate in the reaction tube and a color change after the addition of SYBR Green I dye. Also, the cross-reactivity tests for all non-Classical swine fever viruses were negative (Fig. 3). Finally, the amplified products were confirmed by digestion with restriction enzyme Hinf I (Fig. 4).
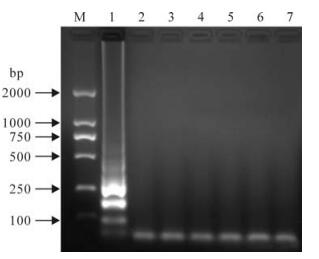
Figure 3. Specificity of the CSFV RT-LAMP method. M, 2000-bp DNA marker; 1, RT-LAMP product of CSFV-Shimen; 2-7 lane, RT-LAMP product of Bovine viral diarrhea virus (BVDV), Respiratory syndrome virus (PRRSV), Swine influenza virus (SIV), Porcine parvovirus (PPV), Pseudorabies virus (PRV) and Porcine circovirus (PCV), respectively.
-
A total of 157 samples were used in this study for comparative evaluation between RT-LAMP and RT-PCR. The detection rates of RT-LAMP and RT-PCR were 26.11% and 14.65% respectively. None of the RT-PCR-positive samples were missed by RT-LAMP. Critically, RT-LAMP identified eighteen additional positive cases that were found to be negative by RT-PCR suggesting a higher sensitivity for this assay (Table 1).

Table 1. Comparative evaluation of RT-LAMP assay with RT-PCR for 157 samples with CSF clinical signs.
Comparison of the sensitivity of RT-LAMP with that of RT-PCR
Specificity of CSFV RT-LAMP assay
Evaluation of CSFV RT-LAMP assay
-
Classical swine fever is an important disease in pigs, which causes significant economical losses in the pig industry worldwide and though some countries had supposedly eradicated CSF, it has re-appeared in some areas and led to the slaughter of millions of pigs[4]. More importantly, the clinical prevalence of apparent infection with CSFV posed a greater risk to the pig industry. However, current methods for the detection of CSFV such as real-time PCR and RT-nest PCR are either time consuming or require special equipment.
The RT-LAMP assay is a useful diagnostic technique with many advantages. Firstly, the RT-LAMP reaction takes place under isothermal conditions so no heating equipment is required: a simple water bath or heat pack can accomplish the amplification. Secondly, the assay is simple. The whole reaction is carried out in a single tube by mixing several reagents together at 63℃ for 1 h. Thirdly, it is efficient. Large amounts of the products and by-products that RT-LAMP yields can be identified visually. Fourthly, the RT-LAMP is a one-step assay in which the reverse transcription and LAMP reaction are combined by adding AMV reverse transcriptase to the same tube, which further simplifies the assay when compared with the conventional RT-PCR method. Therefore, in the field of disease diagnosis the RT-LAMP assay has the potential to replace current nucleic acid amplification techniques [2, 12].
In this study an RT-LAMP assay was established for CSFV detection. A set of six primers was designed against the conserved coding regions in CSFV and strain SM was used for optimization of the assay. RT-LAMP was able to detect 13 strains of CSFV and showed good specificity. RT-LAMP was able to detect a 10-6dilution of RNA extracted from blood with 103.84TCID50CSFV, which was 100-fold more sensitive than RT-PCR. For clinical diagnosis of CSFV, the data presented in this study shows that the RT-LAMP assay is more sensitive than RT-PCR as it identified eighteen additional cases that were found to be negative by RT-PCR.
In conclusion, the RT-LAMP assay provides a simple, rapid, sensitive and accurate method for detection of CSFV in clinical samples. The assay does not require any sophisticated equipment and in many situations is a far more suitable technique for the detection of CSFV in the field.















 DownLoad:
DownLoad: