-
Bluetongue (BT) is an infectious, non-contagious viral disease leading to substantial economic losses to the livestock industry. Bluetongue virus (BTV) is an economically important arbovirus that affects domestic and wild ruminants and various other Artiodactyla[7]. BTV (F: Reoviridae, G: Orbivirus) is a complex, non-enveloped virus with 7 structural and 4 nonstructural proteins encoded by 10 segments of dsRNA and has 24 serotypes[4] with the involvement of Culicoides vectors in its transmission[6]. All the serotypes share a common VP7 protein, but vary in their VP2 and VP5 proteins which produce neutralizing antibody in the infected animals. BTV exhibits a distinct evolutionary lineage or topotype making the diagnosis more difficult.
In field conditions, sheep is the primary host and the tentative diagnosis of BT relies on the disease epizootiology, vector distribution, clinical symptoms and pathological lesions. Clinical symptoms are not sufficient to determine quarantine and implementation of a control program. Isolation of the virus in embryonated chicken eggs[5] or cell cultures[17] is also required, along with identification of the viral antigens in the tissues/body fluids or antibodies in sera by various diagnostic assays such as immunofluorescence, immunoperoxidase staining [2], agar gel immuno-diffusion test (AGID), serum neutralisation test (SNT), fluorescent antibody test (FAT), or enzyme linked immunosorbent assay (ELISA)[1, 10, 15, 16]. Additionally, polymerase chain reaction (PCR) and quantitative PCR (QPCR) for detection of viral nucleic acid[12] as well as serogrouping, serotyping and topotyping of BTV isolates have been reported.
Bluetongue cELISA is one of the OIE prescribed tests for international trade of ruminants, while, bluetongue AGID test is an alternative test [8]. BT is enzootic in India and its occurrence is sporadic. The presence of 21 serotypes in the country has complicated the diagnosis, serotyping and characterization of the virus as well as implementation of a control strategy. Currently, the sero-surveillance and sero-diagnosis of BT is being carried out using imported ELISA kits, which are expensive and often associated with the risk of importing exotic pathogens. In view of the above, the present study was undertaken to produce and characterize monoclonal antibodies against BTV23.
HTML
-
BHK21 cell line (ATCC, Manassas, Virginia) was used for propagation of BTV. Cells were cultivated in tissue culture flasks (Nunc, Denmark) in Glasgow's minimum essential medium (GMEM) supplemented with 10% new born calf serum (NBCS, HyClone, USA) at 37℃. The BTV1, BTV2, BTV15, BTV17, BTV18 and BTV23 serotypes available in India were used in the study. These serotypes were amplified and maintained in BHK21 cell culture and maintained at the Division of Virology, Mukteswar, Nainital (Distt.), Uttarakhand, India.
For bulk production of BTV23, BHK21 cells were grown in roller culture bottles (1700 cm2, M/s Greiner, Germany) to 90% confluency and infected with BTV by adsorption for 30 min. at 37℃. Ten rollers infected with BTV23 were harvested after 72 h post infection (PI) at 80% CPE and pooled. The virus material was purified using discontinuous sucrose density gradient ultra centrifugation. A compact opaque disk was observed above the 66% sucrose layer. The virus band was collected and further cushioned through 40% sucrose. A whitish precipitate of semi-purified virus was obtained at the bottom of the 40% sucrose cushion. The precipitate was re-suspended in Tris-EDTA (TE) buffer after centrifugation in order to remove particulate debris[11]. The purity of the virus was checked spectrophotometrically, quantified and stored at –80℃ until use. The purified virus was used to immunize Balb/C mice for production of monoclonal antibodies.
-
Purified truncated recombinant VP7 protein of BTV23 expressed in E. coli [13] was used to check the specificity of BTV reactive clones.
-
Two adult rabbits were each immunised with 100 µg of BTV23 along with Freund's complete adjuvant (FCA) and then incomplete Freund's adjuvant (IFA) followed by weekly boosters with pure antigen in PBS for 4 weeks. The rabbits were test bled and the presence of antibodies against BTV was confirmed using AGID and SNT. After the last booster, the rabbits were bled; serum was collected and preserved at -20℃ until further use.
The 24 BTV serotype specific sera were obtained from the Office International des Epizooties reference laboratory (Onderstepoort Veterinary Institute, Republic of South Africa) in freeze-dried form. All these sera were raised in guinea pig and were reconstituted in phosphate buffered saline (PBS) and kept at -20℃ until use.
-
Balb/C mice originally obtained from National Institute of Nutrition (NIN), Hyderabad, Andhra Pradesh, India were maintained and bred at the Division of Virology, IVRI, Mukteswar and used in this study. Six Balb/C mice, aged 3-5 months were selected and each mouse was inoculated with 50-100 µg (in a total volume of 200 µL) of purified BTV23 antigen. Mice were inoculated intraperitoneally (i/p) with 100 µg (in 100 µL PBS) of purified BTV23 with 100 µL of FCA. Each mouse was boosted every week with 50 µg (in 100 µL PBS) of antigen emulsified with 100 µL of IFA for two weeks intraperiotneally. Each mouse was boost immunized intravenously with neat antigen @ 50 µg in 200µL PBS on days 28, 29 and 30 post immunisation (dpi). The fusion experiment was carried out on 31 dpi [14].
-
Myeloma cell line SP2/0-Ag14 was expanded a week before the fusion in complete Iscove's modified Dulbecco medium (IMDM) with 20% foetal bovine serum (FBS) (Sigma, USA) so as to obtain 1×108 cells per Balb/C mice spleen on the day of fusion. The myeloma cells were split a day before the fusion, in IMDM with 20% FBS.
-
Adult Balb/C mice were sacrificed and the abdominal skin was removed. Then, 5 mL of 0.34 mol/L (11.6%) sterile-cold sucrose solution was injected intraperitoneally above the symphysis and over the right lobe of the liver. Abdomen was gently massaged and the peritoneal fluid was drawn aseptically and collected in a beaker to obtain ~1.5-3 × 106 macrophages. The cells were collected in a sterile polypropylene tube and washed with saturated media and used within 30 min. of preparation. The cells were put into 96 well cell culture plates and incubated at 37℃ under 5% CO2. The cell growth was observed daily and was prepared two days prior fusion.
-
The immunized mice were sacrificed by cervical dislocation. Spleen was transferred to a sterile petridish filled with sterile serum free Dulbecco's minimum essential medium (DMEM) and teased to obtain single cell suspension by squeezing with angled forceps. Then spleen cells were transferred to a 50 mL conical centrifuge tube filled with sterile complete serum free DMEM and centrifuged for 5 min at 500×g under room temperature and supernatant was discarded. Red blood corpuscles (RBCs) were lysed by re-suspending pellet in 5 mL ammonium chloride solution (0.826 g ammonium chloride, 0.086 g sodium bicarbonate in 100 mL distilled water, pH 7.2-7.4). The pellet was re-suspended and washed as described above. The myeloma cells were harvested in the same way as described for spleen cells. The spleen and myeloma cells were re-suspended in 10 mL complete serum-free DMEM separately. Cells were counted and the viability of each cell suspension was assessed using a hemacytometer and Trypan blue exclusion. On the basis of cell count, IMDM with 20% FBS was prepared and pre-warmed at 37℃ for further use.
-
The fusion of spleen and myeloma cells was carried out in accordance with the standard protocol[3]. Myeloma and spleen cells were mixed at 1:1 ratio in a 50 mL conical centrifuge tube and filled with complete serum free DMEM. Cell mixture was centrifuged for 5 min at 500×g at room temperature. The cell fusion was performed at 37℃ by placing the tube containing the mixed cell pellet in a waterbath maintained at 37℃ in a laminar flow hood. Using a clean pipette, 1mL pre-warmed 50% PEG (Sigma) was added to the mixed cell pellet drop-by-drop over 1 min. Also, with a clean pipette, 1mL pre-warmed complete serum free DMEM was added to the cell mixture drop-by-drop over 1 min. It was repeated once with an additional 1mL of pre-warmed serum-free DMEM. With a 10 mL pipette, 7 mL pre-warmed serum-free DMEM was added drop by drop over 2 to 3 min. Then the cell suspension was centrifuged for 5 min. at 500×g at room temperature. With a clean 10 mL pipette, 10 mL pre-warmed complete IMDM was forcefully discharged to the cell pellet. Then two drops of suspension was added to each well of a 96-well flat bottom plate and incubated overnight in a humidified 37℃, 5% CO2 incubator. Growth of hybridoma was observed regularly under selection medium containing hypoxanthine-aminopterin-thymidine (HAT). Well to well screening of hybridoma clones, employing indirect ELISA was performed at the appropriate stage of the growth.
-
The reactivity of all the hybridoma supernatants was checked with whole virus in indirect ELISA (iELISA). Briefly, the ELISA plates were coated with purified BTV23 antigen (1:50 dilution in PBS, pH 7.4) at 100 µL/well. The plates were incubated at 37℃ for 1 h under constant orbital shaking. Unbound antigen was washed off using 1:4 diluted PBS containing 0.05% Tween 20. Then, 100 µL/well of each culture supernatant of individual clones diluted 1:2 in blocking buffer (PBS + 0.01% Tween 20 + 1% BSA) were added in duplicate wells. Then plates were incubated at 37℃ for 1 h under constant orbital shaking and washed. Anti-mouse HRPO conjugate diluted 1:1000 in blocking buffer was added at 100 µL/well. Plates were further incubated for 1 h as described earlier. Plates were washed off and OPD comprising 30% H2O2 (0.5 µL/mL of OPD) were added to all the wells. Colour reaction was observed for 15 min. followed by stopping the reaction with equal volume of 1mol/L H2SO4. The optical densities were read at 492 nm. The clones that gave significantly higher absorbance (A492) values (at least two times) with purified BTV23 compared to BHK21 antigen were selected for further investigation.
-
Further, all the BTV reactive clones were checked for their specific reactivity with rVP7 protein [13] by employing iELISA as described above. The ELISA plates were coated with rVP7 protein at 100ng/well in lieu of whole BTV antigen and the remaining steps were same as before.
-
Clones giving positive reactivity with BTV and rVP7 in iELISA were amplified and subjected to single cell cloning and sub-cloning using limiting dilution method [14]. Single cell cloning was repeated at least three times for each reactive clone in order to establish the monoclonality of the hybridoma culture supernatant.
-
The isotypes of the 24 hybridoma clones were determined by testing the cell culture supernatant using a commercially available isotyping kit (Sigma-Aldrich, USA) as per manufacturer's protocol. All the supernatants were used either undiluted or diluted 1:2 in buffer for determining their isotype.
-
The ELISA plates were coated with purified BTV23 [1:50 dilution in PBS, pH 7.4] at 100 µL/well or rVP7 at 50 ng/well in 100 µL PBS. The plates were incubated at 37℃ for 1 h under constant orbital shaking. Unbound antigen was washed off using diluted PBS (1:4 in distilled water). In order to determine the antibody titre, two-fold dilutions of hybridoma culture supernatants were prepared in blocking buffer (1% BSA + 0.05% Tween 20 in PBS, pH 7.4) in a separate eppendorf tube. From this, 100 µL/well was added to each designated well. The remaining steps were followed as described earlier. The dilution of the monoclonal antibodies which gave 75% A492 of the plateau (maximum A492) was considered as titre of each monoclonal antibody for practical purposes.
-
Competitive ELISA (c-ELISA) for determining the reactivity of all individual serotype specific sera of BTV with VP7 reactive clones was performed as per Anderson [1] with the following modifications. In brief, a pre-determined dilution of BTV23 purified antigen (1:100, in 100 μL/well) or rVP7 (100 ng in 100μL /well) in PBS (0.01mol/L, pH 7.2) was coated onto 96-well, flat-bottomed, polystyrene micro-titre ELISA plates (Nunc, Germany). Appropriate controls (mono-clonal antibody, positive, negative and conjugate) were maintained. The plates were incubated at 37℃ for 1 h with constant orbital shaking between each step. The plates were washed three times using PBS (1:4). Then, 50 μL blocking buffer containing 1% BSA and 0.05% Tween 20 in PBS was added to all the wells except for A11 to H11 (column 11). In Column 12 (A12 to H12), 100 μL of blocking buffer was added. 50 μL of BTV-1 HIS was serially diluted from 1:1 to 1: 256 from A1 to A10. Similarly, other sera (HIS to BTV2 to 24) were diluted in separate plates in separate rows. Immediately, 50 μL of predetermined dilution (1:7.5) of MAb supernatant, 4A10 diluted in blocking buffer was added in all the wells including MAb control (A11to H11), except conjugate control (A12 to H12). The remaining steps were followed as described earlier. The percentage of inhibition (PI) was calculated as per, PI=100 −[(OD of test sample/OD of MAb control) × 100] equation.
Cell line, viruses and bulk production and purification of BTV23
Recombinant VP7 protein (rVP7)
Hyperimmune serum and bluetongue virus serotype-specific sera
Balb/C mice and immunization
Myeloma cells
Feeder cells
Spleen cells
Hybridoma production
Characterization of MAb
Reactivity of MAbs with BTV
Reactivity of MAbs with recombinant VP7 protein (rVP7)
Single cell cloning and subcloning
Isotyping
Titration of MAbs
Reactivity of selected MAb with serotype-specific sera of BTV in cELISA
-
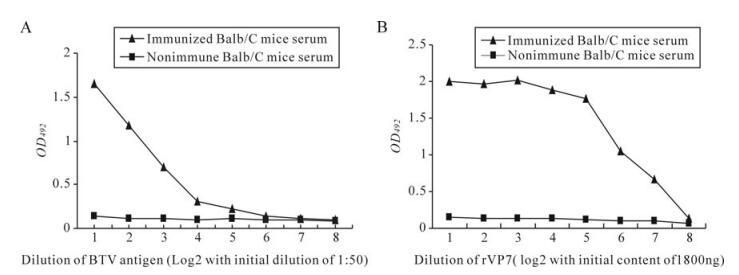
Each of the six BTV serotypes BTV1, 2, 15, 17, 18 and 23 produced specific cytopathic effects (CPE) after 36 h post infection (PI) which was completed after 72 h PI. Furthermore, BTV1, 2, 15, 17, 18 and 23 were also confirmed by polyacrylamide gel electropho-resis (Data not shown). BTV23 was produced in bulk in the BHK21 cell line for further study. The titre of the virus was 106.5TCID50/mL. The protein content of the purified virus was 1.75 mg/mL and the purity of the virus was 90%. The immunized mice were test bled before fusion and tested for the antibodies against BTV in iELISA (Fig. 1).
-
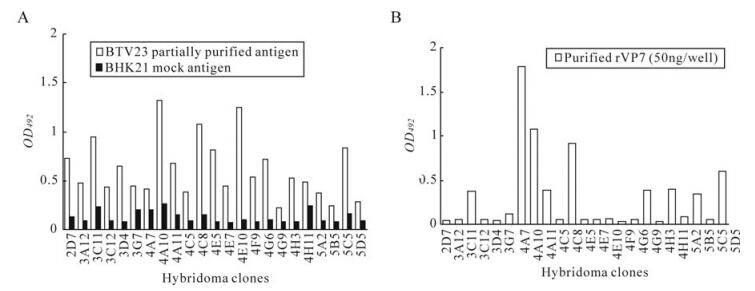
The reactivity of hybridoma culture supernatants with purified BTV23 and BHK21 in iELISAis shown in figure 2A. Similarly, all the BTV reactive clones (n = 24) were further tested for their reactivity with rVP7. Likewise, all the selected primary hybridoma clones were checked for their specificity to rVP7 protein in indirect ELISA. Out of 24 clones, only eight clones (4A7, 4A10, 4A11, 4C8, 4G9, 4H11, 5B5 and 5D5) were found to specifically react with rVP7 protein (Fig. 2B).
-
Of the 24 MAbs isotypes, the majority of them were found to be IgG class belonging to different subclasses. The remainder, 3G7, 4A7, 5D5, 4E5, 4G6, 4G9 belonged to the IgM class. The details of isotypes, reactivity with BTV23, rVP7, titres and their diagnostic applications are shown in Table 1.

Table 1. Characteristics of bluetongue virus specific monoclonal antibodies
-
The titres and titration curves of some of the MAbs are presented in Table Ⅰ and Figure 3. Overall, the MAbs with sigmoid curves (4A7, 5D5 with BTV23 antigen and 4A10, 4A11 and 4H11 with purified rVP7 protein of BTV23) had high antibody titres followed by MAbs with linear curves (4G9 and 5B5 with BTV23 antigen). Low antibody titres were observed in MAbs having concave reactivity curves (4A10, 4A11, 4C8 and 4H11 with BTV23 antigen; 4A7, 4C8, 4G9 and 5B5 with rVP7 protein of BTV23) which reacted only at lower dilution or undiluted form or rarely when diluted 1:2 (Fig. 3).
-
Of the 8 clones tested for their suitability in c‑ELISA, only the clone designated as 4A10 was found to be possibly suitable as inhibition of the rest of the clones was much less than the 4A10 clone with BTV23 hyperimmune serum. All the 24 serotype specific sera of BTV inhibited the MAb with variable percentage inhibition ranging from 16.6% with BTV 12 serotype to 78.9% with BTV16 serotype at the lowest dilution of serum (which indicates the specificity of the MAb). A highest PI of 78.9% was observed with HIS of BTV16 followed by 76.2% with BTV5 serotype and the least was observed with BTV12 (16.6%), at lowest dilution of serum. The control or healthy sera also showed certain level of inhibition at low dilution. The PI was decreased as the dilution of the sera was increased (Fig. 4). Similarly, variable levels of PI were observed with a few of the selected field sera of sheep (Table 2). Epizootic haemorrhagic disease, Ibaraki and Akabane viruses have not yet reported in the country, so the cross reactivity with these viruses has not been ascertained.
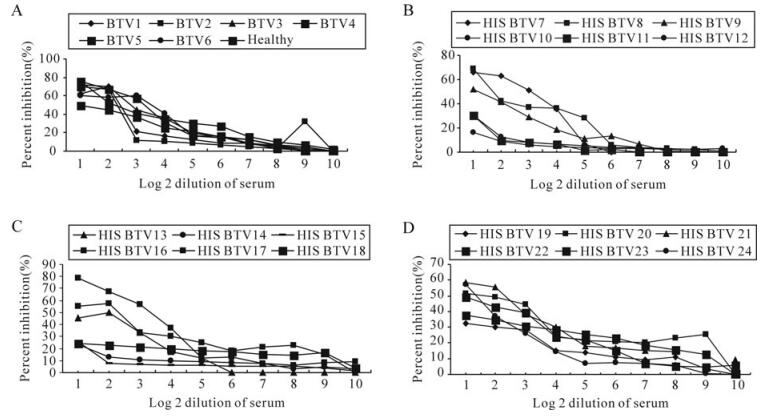
Figure 4. Inhibition of MAb by individual BTV serotype specific serum in c‑ELISA. A: BTV1-6 and healthy sheep serum; B: BTV7-12; C: BTV13-18; D: BTV19-24.

Table 2. Field and representative hyperimmune sera showing variable percentage of inhibition in cELISA using whole BTV virus and rVP7 protein
Identity, bulk production and purification of BTV23
Reactivity of selected hybridoma clones with BTV and rVP7
Isotyping of MAb/s
Titration of MAb/s
Reactivity of selected MAb with serotype-specific sera of BTV in cELISA
-
The present study was designed to produce and characterize the MAb/s specific to BTV and more specifically to the VP7 protein of BTV with a possibility of development of MAb based ELISAs. To do so, BTV23 was purified and used for the immunization of Balb/C mice [14]. A total of three fusion experiments were conducted with and without feeder cells. The fusion experiments with feeder cells performed better than those without feeder cells. Growing clones were noticed as early as 5th day post fusion. A total of 24 BTV reactive hybridoma clones were obtained. This was ascertained using indirect ELISA. The selection was made on the basis of reactivity of clones with purified BTV23 and mock infected BHK21 antigen. The clones, which gave a reactivity twice or more than twice with BTV compared to mock infected antigen (BHK21) were selected, grown and limiting dilution was performed in order to obtain the monoclonality. Further, the clones were characterized for their reactivity with whole virus, the rVP7 protein, their titres, and isotypes. All the 24 clones were checked for their group specificity with rVP7 in iELISA. Out of 24 BTV clones, only eight clones reacted with rVP7, suggesting their group specificity and the rest of the clones might be directed against other proteins of BTV including VP2 and VP5 as the whole virus was used for immunization. The clones reacted variably with the whole virus and rVP7 which may be because of the variation in their titres. Some clones had high antibody titre and some had very low antibody titre, this may also be the reason for variable reactivity of clones in iELISA. The shape of the graph is an indication of titre of a particular MAb. Later, all the clones were subjected to isotyping using a commercial isotyping kit. The majority of the MAbs were of IgG type and a few were of the IgM class. Similarly, isotyping of BTV reactive MAbs was done elsewhere and the results were consistent [15].
The specificity of the clones was further ascertained using recombinant VP7 protein; mock infected antigen, BTV23 whole virus, goatpox virus and sheeppox virus in iELISA. Based on their reactivity, clones may be designated as group-specific, type-specific or cross-reactive clones. Out of 24 clones, only eight clones (4A7, 4A10, 4A11, 4C8, 4G9, 4H11, 5B5 and 5D5) reacted variably but specifically with the rVP7 protein of BTV, establishing the fact that these clones were directed to the epitopes of the VP7 protein and therefore these clones had potential use in diagnostic assay/s. Cell culture supernatants of these clones were also tested with BTV serotypes 1, 2, 15, 17, 18 and 23 to re-establish their group specificity (Data not shown). However, these clones might have been directed to different epitopes of the VP7 protein which may be the reason for variability in their reactivity with the whole virus and rVP7.
Although all the clones reacted well in iELISA, not all of them were suitable for cELISA. Only the 4A10 clone was found to be possibly suitable for c‑ELISA. The 4A10 clone showed variable PI with guinea pig hyperimmune sera against all 24 tested serotypes of BTV and also with sheep anti-BTV sera raised against BTV serotypes 1, 2, 15, 17, 18 and 23 in our laboratory. The PI ranged from 16.6 to 78.9% for the BTV12 and BTV16 serotypes, respectively. The PI for other serotype-specific sera lies in between this range. The low rate of inhibition with BTV12 may be partly due to unavailability of the epitope recognised by the MAb and may be a difference in the group antigen of BTV12 [9]. It could be also caused by low titre of antibody in the serum. This finding is in accordance with previous studies, where BTV19 antiserum consistently gave low percent of inhibition even though the serum was rich in antibodies to BTV as shown by indirect ELISA [1]. In the present study, BTV23 was used for raising the MAbs, while, earlier research used BTV1 [1]. Some field serum samples were also tested for their ability to inhibit the MAb in cELISA using whole virus and rVP7 separately as coating antigen. Some amount of inhibition was also observed with healthy sheep serum and this could be due to the presence of low level of antibodies to BTV as the serum was tested initially using AGPT. So the above results indicate that the clone 4A10 was group specific and so has diagnostic potential. In conclusion, the MAbs produced against BTV were characterized and their possible suitability in cELISA was ascertained. However, further purification of MAb and validation of the test are needed. The MAbs produced against BTV are likely to be useful for immunohistochemical studies and for developing appropriate pen-side diagnostic tests such as a chromatographic strip test, a latex agglutination test or lateral flow assay as these tests are easy to perform, simple and rapid.















 DownLoad:
DownLoad: