-
Foot-and-mouth disease virus (FMDV) is a member of genus Aphthovirus of the family Picornaviridae and causes the highly contagious vesicular disease foot-and-mouth (FMD) of cloven-hoofed animals [10, 21]. Unlike cattle and pigs, FMD in sheep is frequently mild or non apparent, so that subsequent transmission to other susceptible species can produce devastating consequences [13, 9]. Thus sheep can be considered an important target species for FMDV vaccines. Although traditionally inactivated vaccines play a key role for control and eradication of the disease in some parts of the world, these chemically inactivated vaccines have several disadvantages including the need to store under refrigeration [1] and difficult to discrimination between infected and vaccinated animals [14, 16]. Additionally, there is a potential risk of live virus escaping from vaccine plants [1, 2, 13].
Epitope vaccines againt FMDV are a novel vaccine and represented one of the safest methods for eliciting neutralizing antibodies to FMDV and confering complete protection in small animals [20, 23]. However, this vaccine only could offer limited protection in the natural hosts [17, 22, 23]. This may be due to the poor immunogenicity of antigenic epitopes and the possible lack of the appreciate T-helper cell epitopes [3, 8]. Previous reports have indicated that the host-self immunoglobulin G heavy constant region as a potential protein carrier could not only significantly improve the immunogenicity of antigenic epitopes, but could also reduce side-effects compared with that of other foreign protein carriers [7, 24, 25].
In this study, to develop a promising multiple-epitope vaccine against FMDV type Asia 1 in sheep, a tandem repeated multiple-epitope vaccine coupled with the ovine IgG heavy constant region gene was developed. The potential of the multiple-epitope recombinant vaccine was evaluated in guinea pigs and sheep. The results showed that the multiple-epitope recombinant vaccine could elicit high titers of neutralizing antibodies to FMDV in guinea pigs, and conferred full protection in guinea pigs against virus challenge with 103 GPID50 of FMDV. Particularly, the recombinant protein RE-oIgG was able to elicit protective levels of neutralizing antibodies in sheep. We speculated that this multiple-epitope recombinant vaccine is a promising vaccine regime which may be used for control and eradication of FMDV in the future.
HTML
-
Amplification of ovine IgG heavy constant region gene
mRNAs extracted from ovine spleen using an RNeasy kit (Qiagen) were used for the ovine IgG template for RT-PCR. oIgG was amplified by PCR with the specific primers forward primer 5'-GAATTCATGAACCCACTGTGGACCCTCCTCTTTGTG-3' (EcoR Ⅰ) and reverse primer 5'-CTCGAGTTTACCC GCAGACTTAGAGGTGGACTTCTG-3' (Xho Ⅰ). RT-PCR was performed by one-step RNA PCR kit according to the manufacture's instructions (Takara, Japan). Briefly, 50.0 μL of reaction mixtures contained 5.0 μL of 10 × one-step RNA PCR buffer, 10.0 μL of MgCl2 (25 mmol/L), 5.0 μL of dNTPs (10 mmol/L each), 1.0 μL of RNase inhibitor, 1.0 μL of AMV RTase XL, 1.0 μL of AMV-Optimized Taq and 1.0 μL of each primer (20 pmol), 10.0 μL of template mRNA and 16.0 μL of distilled water. The first strand cDNA was synthesized at 50℃ for 30 min, and subsequently incubated at 94℃ for 5 min. PCR was then carried out for 30 cycles at 94℃ for 1 min, 56℃ for 30 sec and 72℃ for 90 sec, followed by a final extension for 8 min at 72℃.
Design and synthesis of a tandem repeated multiple-epitope gene RE
Based on the VP1 sequence of the FMDV type Asia 1 China/JS/05 isolate (GenBank accession number, EF149009), two antigenic epitopes corresponding to residues 135-160 and 198-211 of VP1 of FMDV type Asia 1 were chosen for the immunogens, and then a tandem repeated multiple-epitope gene (RE) was synthesized by sequential linking of the tandem repeats. To minimize interference between adjacent epitopes and to avoid development of a new epitope, a linker sequence, GGSSGG, was used to separate adjacent epitopes. The RE gene was synthesized artificially by overlapping PCR and cloned into the vector pUC-18 to produce pUC-18-RE (Fig. 1).
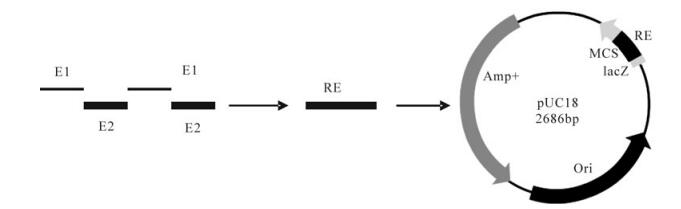
Figure 1. schematic representaion of tandem repeated multiple-epitopes gene and constructuon of the recombinant plasmid PUC-18-RE used in this study
Construction of the recombinant expression plasmids
Recombinant expression plasmids, pET-30a-RE and pET-30a-RE-oIgG, were constructed. Briefly, RE gene was cloned into the expression vector pET-30a (+) pre-linearized with BamH Ⅰ/EcoR Ⅰ (NEB), which resulted in a recombinant expression plasmid pET-30a-RE. pET-30a-RE-oIgG was created by cloning the oIgG gene into the C-termunis of the RE gene in the recombinant plasmid pET-30a-RE. All positive recombinant plasmids were sequenced to confirm the target genes were inserted correctly in the recom-binant open reading frame.
Expression, purification and characterization of the recombinant proteins
Expression and purification of the recombinant proteins in E. coli BL21 (DE3) cells (Novagen) was performed as described previously [15] with slight modifications. Briefly, the transformed bacteria were grown in 100 mL of LB medium supplemented with 50 μg/mL of Kalamycine at 37℃ overnight in a shaker. Growth was monitored by absorbance mea-surements at 600 nm (OD600) and at OD600 = 0.4-0.6, expression was induced with 0.4 mmol/L of IPTG. Cells were grown for a further 6 h at 37℃ and harvested by centrifugation. The cells were sonicated on ice (10 × 30 sec). The supernatant was collected and incubated with Ni-NTA agarose resin (Qiagen) (2.5 mL of resin for 1 liter of induced cells) equilibrated in Buffer A (25 mmol/L Tris, pH 8.0, 100 mmol/L NaCl, 1 mmol/L 2-ME, 0.1% Triton X-100) with 6 mol/L urea, and then stirred for 1 h to allow binding. The resin was washed with 10 column vol of Buffer A and 3 column vol of 160 mmol/L imidazole. Fractions of the eluate were analyzed on 12% SDS-PAGE. The concentration of each protein was determined by Bio-Rad Protein Assay according to the manufacturer's instruction (Bio-Rad).
Western blotting analysis of the recombinant proteins
The immunoreactivity of each protein was determined by Western blotting. Briefly, the purified protein was run separately on 12% SDS-PAGE, and then transferred to a PVDF membrane. The membrane was blocked by phosphate-buffered saline with 10% horse serum for 1 h, and then washed 3 times with PBST. The membrane with the target proteins, RE and RE-oIgG, was incubated with a 1:500 (v/v) dilution of the positive serum from cattle infected with FMDV for 1 h and washed three time with PBST, and then incubated with a 1:2 000 dilution of rabbit anti-cattle IgG antibody conjugated with horseradish peroxidase (Sigma) for 1 h. After five washes with PBST, signals were developed with 3, 3'-Diaminobenzidine tetrahy-drochloride (DAB, Sigma).
Challenge virus
FMDV type Asia 1 China/JS/05 isolate was provided by the National Foot-and-Mouth Disease Reference Laboratory of the P. R. China. Virus was propagated for 5 passages in guinea pigs and a titeration of 50% guinea pigs infective dose (GPID50), 105.75 GPID50, was determined.
Potency of immunity of the epitope vaccine in guinea pigs
The optimized dose of vaccine was determined previously (Data not shown). The immunogens, RE and RE-oIgG, were adjusted to appropriate concentration (200 μg/mL), and then separately emulsified in an equal volume of Montanide ISA 206 adjuvant (France). Twenty female guinea pigs weighing 250-300 g were randomly divided into 4 groups. Group 1 was vaccinated intramuscularly with 1 mL of PBS in oil emulsion. Group 2 was vaccinated with 1mL of RE. Group 3 was vaccinated with 1mL of RE-oIgG. Group 4 only received 1 mL of the inactivated vaccine. All animals were booster vaccinated on day 28 after initiation vaccination. Animals were bled individually by the heart puncture at days 0, 28 and 42 post-vaccination (dpv), where 42 represents 14 days after booster vaccination. All serum samples were used for detection of neutralizing antibodies by micro-neutralization assay.
All guinea pigs were challenged intradermally in the foot pad with 103 GPID50 of FMDV type Asia 1 China/JS/05 in 0.2 mL PBS at day 14 after booster vaccination. The characteristic FMD lesions were monitored daily for 7 days and protection was determined as described previously [6].
Immune responses in sheep
Fifteen sheep weighing 20-25 kg, free of antibodies to FMDV ( < 1:4) and nonstructural proteins of FMDV were chosen and equally divided into three groups. Groups 1-3 were vaccinated intramuscularly with 1 mL of PBS in oil emulsion, RE and RE-oIgG, respectively. All sheep were booster vaccinated with an equivalent dose on day 28 after first vaccination. Serum samples were collected at 28 and 42 dpv, respectively. The levels of neutralizing anibodies were detected by micro-neutralization assay.
Virus neutralizing assay
Neutralizing antibodies assays were carried out in 96 wells as described previously [12]. End-point titres were calculated as the reciprocal of the last serum dilution to neutralise 100 TCID50 of homologous FMDV in 50% of the wells.
Lymphocytes proliferation assay
The lymphocyte proliferation was analyzed by flow cytometry as described previously [11]. Briefly, heparinized whole blood was collected from the vaccinated guinea pigs groups 1-4 at 42 dpv. The peripheral blood mononclear cells (PBMCs) were selected by centrifugation over Ficoll-Hypaque (density 1.007 × g/L). Cells were resuspended at a final concentration of 5 × 107 cells/mL in RPMI-1640 and stained with 2 μmol/L of CFSE (Sigma) for 15 min at 37℃, and then five volumes of ice-cold RPMI-1640 with 10% FBS was added to quench staining for 5-10 min at room temperature. Finally, cells were resuspended at 1.5 × 106 cells/mL in complete RPMI-1640 medium [10% FCS, 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, 10 mmol/L Hepes, 50 mmol/L β-ME, ampicillin (100 IU/mL) and steptomycin (100 μg/mL)], and dispensed into a 96-well tissue culture plate together with 10 μg of purified FMDV antigen (type Asia 1) and 5 μg/mL of ConA (Sigma). The negative control received only the culture medium, 10 μg/mL of BSA (Sigma), as a non-correlated antigen control. Cultures were incubated for 72 hours at 37 ℃ in an incubator with 5% CO2. The proportions of lymphocytes proliferation were determined using a FACS Calibur (BD Biosciences).
-
Characterization of the recombinant antigens
The sequencing results showed that all recom-binant expression plasmids were constructed succes-sfully and the chimeric genes were inserted correctly in the plasmid open reading frame. The recombinant proteins, RE and RE-oIgG, were specifically expressed as a formation of inclusion bodies in E. coli, and the specific band could be clearly visualized on an 12% SDS-PAGE gel (Fig. 2). Western blotting showed that the recombinant proteins, RE and RE-ocIgG, could react specifically with the anti-FMDV positive sera (type Asia 1) (Fig. 3). Although the recombinant protein was expressed as a formation of inclusion bodies, our experiments showed that the denatured protein did not affect the immunogenicity and the function of IgG. This result was confirmed in other experiments in our laboratory [19].
Figure 2. The expression analysis of the recombinant proteins in E.coli cells. A: Expression of the recombinant protein RE-oIgG. B: Expression of the recombinant protein RE. Lane 1-3. standard protein marker, before and after induction with IPTG respectively
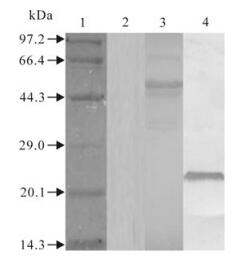
Figure 3. Western blot of recombinant proteins RE-oIgG and RE. Lane 1-4, Standard protein marker, negative control, recom-binant protein RE-oIgG and RE, respectively
Potency of the recombinant vaccine in guinea pigs
As shown in Fig. 4, the recombinant protein RE-oIgG elicited significantly higher titers of neutralizing antibodies in vaccinated guinea pigs (t-test, p < 0.01) compared to the RE recombinant. Furthermore, RE-oIgG was able to elicit a level of neutralizing antibodies as high as that induced by the traditional inactivated vaccine (t-test, p > 0.05). Neutralizing antibodies to FMDV still remained negative in a control group.
Figure 4. Profile of neutralizing antibodies to FMDV type Asia 1 in guinea pigs prior to or after vaccination. PBS, The negative control; RE, The recombinant protein RE; RE-oIgG, The fusion protein of RE and oIgG. Ⅳ, The traditionally inactivated vaccine
Inspiringly, as shown in Table 1, all guinea pigs vaccinated with either a commercial vaccine or the recombinant protein RE-oIgG were completely protected against the challenge. Although RE could not offer protection in guinea pigs, it could delay the appearance of clinical signs and reduce the severity of disease. The negative control group was completely susceptible and developed the typical vesicles at hours 24 after challenge.

Table 1. The results of the vaccinated guinea pigs challenged with FMDV type Asia Ⅰ
Lymphocytes proliferation assay
As shown in Fig. 5, higher percentages of lymphocytes proliferation were obtained from RE-oIgG, which was significantly higher than that observed for recombinant protein RE (p < 0.01, t-test). Inspiringly, the recombinant protein RE-oIgG was able to elicit the same level of lymphocytes proliferation as induced by the traditionally inactivated vaccine (p > 0.05, t-test). In addition, the negative control and non-correlated antigen BSA did not induce significant proliferation responses.
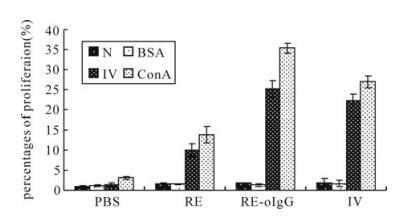
Figure 5. Profile of lymphocytes proliferation in PBMCs selected from the vaccinated guinea pigs on day 42 postvaccination. N, negative control; BSA, A non-correlated antigen control; RA, recombinant anitigen. Ⅳ, inactivated whole virus antigen; ConA, nonnspecific stimulator
Immune response in sheep
As shown in Fig. 6, the higher titers of neutralizing antibodies were elicited in sheep vaccinated with RE-oIgG. A significant difference in the titers of neutralizing antibodies was found between RE and RE-oIgG. However, the negative control PBS maintained negligible antibody responses in the trial period.
-
Humoral immune responses against FMDV are the dominant protective immune response. Particularly, protective neutralizing antibodies are crucial for control and eradication of virus infection [21, 18]. Although the traditionally inactivated vaccines usually elicit high levels of neutralizing antibodies that are correlated with protection against the homologous and antigenically related virus [10, 21] a number of countries with large livestock industries have abandoned vaccination due to the disadvantages mentioned above. In recent years, new generation vaccines, including the epitope vaccines, have been intensively investi-gated [18]. However, the poorly immune potency of novel vaccines in host species limits their use in field.
In this study, the immune stimulator fragment of IgG could not only significantly improve the titers of neutralizing antibodies and induce strong lymphocytes proliferation responses, but could also confer complete protection in guinea pigs after challenge. We speculate that the antigenic epitopes in this tandem repeated gene were sufficiently exposed and the mimicked the naive antigenicity due to introduction of an appropriate spacer between adjacent epitopes. In addition, the IgG heavy constant region as a promising epitope "carrier" could not only increase the molecular mass of short peptides, but also improve capability of the peptides delivery to major histocompatibility complex (MHC) molecules in situ. Previous reports have indicated that antigenized self IgG molecules as a natural long-lived carrier could efficiently delivery peptides to MHC class Ⅱ molecules on the surface of APC, prolonging the half-life of short peptides and reducing the introduction of side effects [4, 5, 7,]. This mechanism of peptide presen-tation may harness the immune responses in vivo by engagement of APCs with a low capacity for antigen processing. In addition, although the high potency of immunity was possibly induced by a misfolded protein since the purified recombinant protein was a formation of inclusion bodies, this denatured protein did not affect its immunogenicity in the test animals. Therefore, we speculated that this vaccine regime is a promising novel vaccine, which may be used for control and prevention of FMD in the future.
Acknowledgments
We thank Professor Juan Chen and assistant-professor Wei-min Ma for technical assistances. This work was supported by the National Science and Technlogy Pillar Program (No. 2006DAD06A03), National High Technology Research and Development Program of China (No. 2006AA10A204), and key project of science and technology of Gansu province (No. 092NKDA030).














 DownLoad:
DownLoad: