-
SARS-coronavirus (SARS-CoV) was confirmed to be the etiological agent of severe acute respiratory syndrome (SARS), the first emerging human disease encountered in the 21st century [8]. It is reported that SARS infection in humans is predominantly associated with mucosal transmission through lungs, intestine or even genital tract [20, 21, 26, 38, 40]. The appropriate anti-SARS-CoV antibody response, especially at diverse mucosal surfaces, would play a crucial role in protection against mucosal transmission of SARS-CoV. For example, the passive transfer of neutralizing serum antibody to naive mice prevented SARS-CoV replication in the lower respiratory tract following intranasal challenge [35]. Prophylactic administration of the neutralizing human monoclonal antibody reduced replication of SARS-CoV in the lungs of infected ferrets, preventing the development of SARS-CoV induced macroscopic lung pathology [39]. Besides, mucosal secretory IgA in the lower respiratory tract, immune protection in the digestive tract and other mucosal sites seems to be crucially important given the fact that transmission of SARS-CoV occurs by direct contact with droplets of the virus by the fecal or oral routes [37]. Taken together, these reports suggest that a wide range of humoral immunity in systemic and diverse mucosal surfaces may play an important role in vaccine-induced protection against SARS-CoV transmission.
Mucosal immunity plays an important role in prevention of invasion by pathogens, which could be induced via systemic or mucosal immunization. However, both immunization protocols require an effective adjuvant to produce better immune responses. Mucosally delivered antigen in the absence of an adjuvant usually induces either a low or undetectable antigen-specific immune response or results in immune tolerance [7, 43]. At present, a few mucosal vaccines have been approved for human use in the United States or elsewhere. Examples include oral vaccines, which mostly comprise live attenuated antigens, against Poliovirus [29], V. cholerae, Salmonella typhi [22], and Rotavirus [14], and a nasal vaccine against influenza virus [3]. It is becoming increasingly apparent that an effective mucosal adjuvant is a high priority in the development of efficient mucosal vaccine.
A synthetic dsRNA, termed Poly(I:C), has been found be able to boost immune responses since the 1960s [10, 45]. Recently, Poly(I:C) was discovered to function as an adjuvant through its interaction with TLR3, which in turn activates the NF-κB pathway, resulting in stable maturation of DCs, activation of NK cells and longer-time survival of activated CD4+ T cells in vitro [42]. Ichinohe et al. [11] reported that intranasal vaccination of hemaglutinin (HA) adjuvanted with Poly(I:C) can induce protection against influenza viral infection in mice. Brian R. Sloat also showed that nasal immunization with anthrax PA plus Poly(I:C) can enhance the production of mucosal and systemic immunities [34]. Therefore, Poly(I:C) represents another potential adjuvant. However, Poly(I:C) itself is unsuitable as a potential adjuvant because it is toxic and can be rapidly hydrolyzed in humans when used alone [6]. Several Poly(I:C) derivatives have been suggested, such as Poly(ICLC), which is Poly(I:C) containing Poly-L-lysine and carboxymethylcellulose [2], to minimize observed deficiencies of Poly (I:C).
PIKA is a particular derivative of Poly(I:C) comprising kanamycin and calcium chloride [25]. A phase I clinical trial with inactivated rabies vaccine in 20 healthy volunteers demonstrated that the PIKA adjuvanted rabies vaccine elicited an earlier detectable and higher neutralizing antibody titer. The PIKA adjuvanted vaccine was well tolerated. A study showed the parenteral adjuvant effect of PIKA in the induction of innate immune responses and HBsAg-specific IgG and IFN-γ responses in mice [33]. Most recent studies also showed that PIKA can provide broad-spectrum prophylaxis against a number of influenza A viruses and coadministration of PIKA with a poorly immunogenic H5N1 subunit vaccine can lead to antigen sparing and quantitative and qualitative improvements of the immune responses over those achieved with an unadjuvanted vaccine in mice [18, 19]. In the present study, we demonstrated that the intraperitoneal and intranasal co-administration of this improved Poly (I:C) derivative induced strong anti-SARS-CoV mucosal and systemic humoral immune responses with neutralizing activity against pseudotyped virus.
HTML
-
SARS-CoV supplied by Wuhan Institute of Biological Product [24] was inactivated with formaldehyde. PIKA is an improved adjuvant formulation comprising a derivative of Poly(I:C), kanamycin and calcium chloride [23, 33], which was manufactured in compliance with Good Manufacturing Practices by NewBiomed PIKA Pte Ltd, Singapore.
-
Female BALB/c mice, 6 to 8 weeks of age, were purchased from Hubei CDC and maintained in SPF environment throughout the experiment. The mice were immunized through either an intranasal (i.n.) or intraperitoneal (i.p.) route with 10 µg of inactivated SARS-CoV or 10 µg of inactivated SARS-CoV plus the indicated dose of PIKA. Mice immunized with PBS served as a negative control. The protocol for intranasal immunization required the mice to be anesthetized slightly with sodium pentobarbital and held inverted with nose up until droplets of vaccine that were applied to both external nares were completely inhaled [13]. All intranasally immunizing reagents were suspended with PBS. Individual mice received 10 µL of inoculants for five times with a 30 min resting interval between inoculations (total volume, 50 µL). For intraperitoneal immunization, vaccine compositions were adjusted with PBS to a consistent 200 µL dose. Each mouse received three immunizations with a 2-week interval between each immunization.
-
All samples were collected two weeks after the final immunization as previously described [9]. Blood samples were collected by retro-orbital plexus puncture. Saliva was obtained after intraperitoneal injection with 20µg of carbamylcholine chloride. The vaginal tract of individual mice was washed with 30µL PBS for three times, with a total volume of 90µL. After removing feces, small intestine tissues were weighed, cut into small pieces, mixed with PBS (100 mg of small intestine in 200µL of PBS), and rocked at 4℃ for 5 h. For collection of lung washing fluid, the trachea was exposed surgically and the oral portion of trachea was clamped, a 1-mL syringe equipped with a 22-gauge needle was inserted into the trachea, 200µL of PBS was injected into the peripheral airways, and five cycles of aspiration and injection were repeated [36]. After centrifugation at 7 000 ×g for 15 min, supernatants of all samples were collected and stored at -80℃ before antibody detection.
-
The anti-SARS-CoV titer of each sample was determined by ELISA. The specific antibodies in serum and mucosal samples were determined by ELISA using the following procedure: microtiter plates were coated at 37℃ for 2 h with 100µL of 3 µg/mL inactivated SARS-CoV diluted with 0.05 mol/L carbonate buffer. After washing, the plates were blocked with 250 µL blocking buffer (1% bovine serum albumin (BSA) in PBS) by incubating at 37℃ for 2 h. Then diluted serum or mucosal samples were added to the plates and incubated overnight at 4℃. After washing with PBST (PBS+0.1% Tween-20), goat anti-mouse IgG-AP (Southern Biotechnology) was used to detect specific IgG and goat anti-mouse IgA-AP (Southern Biotechnology) was used to detect specific IgA in serum and mucosal samples (vagina, saliva, lung and intestine washing fluid). Color was developed with pNPP (Sigma). Optical density at 405 nm was read with an ELISA reader (Thermo Labsystems). The antibody titer was defined as the highest sample dilution that resulted in an absorbance value at least twice that of the counterpart from PBS-immunized mice. Antibody titer for a group of mice was expressed as the arithmetic mean titer ± standard error (SE).
-
The neutralization test was carried out with pseudotyped SARS-CoV produced as Qu et al. described previously with a slight modification [31]. Briefly, one 10-cm-diameter dish of subconfluently grown 293T cells were transfected with 12 µg of pNL.4.3.Luc.E_R_ (HIV-luc) and 12 µg of S gene by using a calcium phosphate transfection method. The medium was replaced 8 h after transfection. Pseudotype vector-containing supernatants were harvested after an additional 40 h incubation; filtered through a 0.22 µm-pore-size Millex-HV filter (Millipore, Schwalbach, Germany); then stored at -80℃ prior to use. For the neutralizing test, Hela-ACE2 cells (104 cells/well) were added to 96-well plates 18 h before infection. Tested samples including serum, saliva, genital tract washing fluid, intestine washing fluid and lung washing fluid were pooled, heat inactivated (56℃, 30min) then serially diluted in Dulbecco's modified Eagle medium (DMEM) containing 10% FBS and a final volume of 30µL was mixed with 30µL of diluted supernatants of pseudotyped SARS-CoV. The virus-sample mixtures were incubated at 37℃ for 1 h and then added to Hela-ACE2 cells. Four hours later, 250µL of fresh medium was added after washing two times with fresh PBS. On 60 h post infection, the infected cells from triplicate wells were lysed for the measurement of luciferase activity according to the manufacturer's instructions (Promega). The neutralizing antibody titer was defined as the dilution of the tested samples that reduced luciferase activity to 50% of the values from the negative control group (immunized with PBS).
-
The western blot assay was carried out according to the protocol in Molecular Clone [32]. Briefly, a segment of spike (S) protein (401~750aa) of SARS-CoV, which was expressed in vitro and named S' [27], was separated by SDS-PAGE on a 5% stacking polyacryl-amide gel in combination with a 12% separating gel. The separated proteins were then transferred to a nitrocellulose membrane for 2 h with a 300 mA current. After blocking with 3% BSA in PBS for 2 h at 37℃ the membranes were incubated overnight at 4℃ with different kinds of serum including; mouse antiserum at a 1:500 dilution, normal mouse serum at a 1:500 dilution for negative control, human antiserum from a convalescent SARS patient at a 1:4 500 dilution for positive control, normal human serum at a 1:4 500 dilution for negative control. After washing 4 times with 0.1% PBST, the membranes were in-cubated with goat anti-mouse (or human)-IgG-AP (1:2000 dilution) for 45min at 37℃. Color was developed with BCIP/NBT after washing. The specific IgA in the intestine washing extract was also determined by western blot, with a 1:40 of sample dilution.
-
The statistical significance of the difference between groups was calculated by student's t-test for two groups or by Tukey's test using ANOVA for three or more groups. Differences were considered not significant when p > 0.05.
Adjuvant and inactivated SARS-CoV
Immunization protocol
Sample collection
End point antibody titers of anti-SARS-CoV
In vitro SARS-CoV Neutralization assay
Western blot
Statistical analysis
-
The optimal concentration of the PIKA adjuvant was identified by immunizing mice intraperitoneally or intranasally with 50 µg, 100 µg, or 250 µg of PIKA mixed with 10 µg of inactivated SARS-CoV antigen. Inactivated SARS-CoV antigen alone (10 µg) was used as a no adjuvant control. The anti-SARS-CoV IgG antibody responses in serum and the IgA antibody in lung tissue were determined by ELISA. As shown in Fig. 1, groups that received 100 µg and 250 µg of the PIKA adjuvant elicited much higher IgG antibody responses in serum than the groups immunized with the antigen alone or the antigen plus 50 µg PIKA, either by intraperitoneal or by intranasal routes of immunization (p < 0.05) (Fig. 1A). However, the IgG antibody responses in serum from groups receiving 100 µg or 250 µg of PIKA showed no significant difference (p > 0.05). While all groups of mice immunized intraperitoneally showed no IgA antibody response, the group of mice immunized intranasally with 100 µg PIKA as the adjuvant showed a significant IgA response in lung. The group of mice immunized intranasally with the higher dose of 250 µg PIKA showed an equivalent level of IgA response in lung (p > 0.05) as the group receiving 100 µg of PIKA (Fig. 1B). Other tests showed similar results (data not shown). Thus, 100 µg of PIKA was chosen as the optimal adjuvant dose in the following experiments.

Figure 1. Specific serum IgG (A) and lung IgA (B) responses in mice adjuvanted with different doses of PIKA. To evaluate the optimal concentration of PIKA as an adjuvant, mice were immunized either intraperitoneally (i.p.) or intranasally (i.n.) with inactivated SARS-CoV alone (10µg), or SARS-CoV (10µg) plus different concentrations of PIKA (50µg /dose, 100µg /dose, 250µg /dose). All mice were immunized three times, and then serum and lung tissues were collected 2 weeks after the last immunization. SARS-CoV-specific serum IgG and lung IgA titers were determined in diluted samples using ELISA. Each bar represents the arithmetic OD value of individual group ± SE. The statistical significance of the difference between groups was calculated by Tukey's test.
-
Neutralizing antibodies are the key components in the protective immune response to SARS-CoV infection because they can bind to viral particles and block them from entering the host cells. We first tested the adjuvanticity of PIKA in inducing neutralizing activity in pooled serum with an in vitro neutralization assay based on the SARS-CoV pseudovirus system. As shown in Fig. 2A, a high neutralizing titer in serum (about 1:477) could be detected in mice intraperitoneally immunized with inactivated SARS-CoV antigen alone. About twenty-five times higher serum neutralizing antibody titers could be induced in mice by co-administration of inactivated SARS-CoV antigen with PIKA (p=0.015). Intranasal immunization of inactivated SARS-CoV antigen with PIKA could also induce neutralizing antibody (1:167 titer) in serum. But intranasal immunization of inactivated SARS-CoV antigen alone could not induce detectable neutralizing activity in serum.
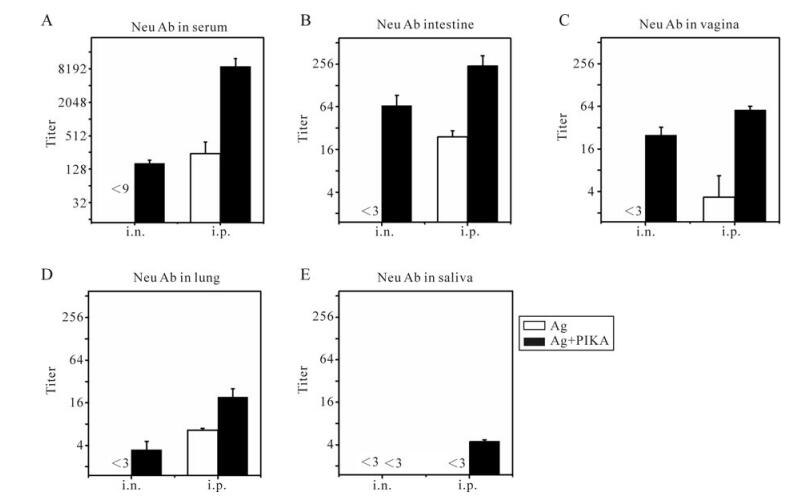
Figure 2. Neutralizing antibodies against SARS-CoV in serum and mucosal samples. Mice were immunized with inactivated SARS-CoV antigen alone or antigen plus PIKA through either intranasal (i.n.) or intraperitoneal (i.p.) route to detect the adjuvanticity of PIKA in inducing neutralizing antibody in serum and mucosal sections. Two weeks after the last immunization, serum (A), intestine washing fluid (B), vagina washing fluid (C), lung washing fluid (D) and saliva (E) were collected and pooled individually to perform a neutralizing assay. Each bar represents the mean ± SE of triplicate parallel wells. Two independent tests were done to confirm the results. Samples from mice immunized with PBS were used as a control. The white bar is the group immunized with antigen alone, while the black bar is the group immunized with antigen plus PIKA. Ag, 10µg of inactivated SARS-CoV alone; Ag+PIKA, 10µg of inactivated SARS-CoV plus 100µg of PIKA.
Given the fact that SARS-CoV can initiate its infection at diverse mucosal sites, we also tested the neutralizing activity in pooled mucosal samples, including lung washing fluid, intestine washing fluid, vagina washing fluid and saliva. Intranasal (i.n.) immunization of inactivated SARS-CoV antigen together with PIKA as an adjuvant was effective in eliciting neutralizing activity, with titer of 1:66.5, 1:25 and 1:3.5 in intestine (Fig. 2B), vaginal washing fluid (Fig. 2C) and lung washing fluid (Fig. 2D) respectively. However, there was no undetectable neutralizing activity in any of the three mucosal samples from the mice i.n. immunized with inactivated SARS-CoV antigen alone. Interestingly, intraperitoneal immuni-zation with only antigen could induce detectable neutralizing activity in intestine (Fig. 2B), vagina (Fig. 2C) and lung (Fig. 2D) mucosal samples, the same as observed in serum (Fig. 2A), which suggested that the route of immunization plays a role in the induction of neutralizing activity. When PIKA was administered intraperitoneally together with antigen it induced a much higher mucosal neutralizing activity compared to mice immunized with antigen alone (Fig. 2B-D) (p < 0.05), which showed the significant adjuvant effect of PIKA. For saliva, no detectable neutralizing activity could be detected in mice intranasally immunized with antigen alone or plus PIKA as an adjuvant.] In mice interperitoneally immunized with antigen alone, while low neutralizing activity could be detected in mice interperitoneally immunized with antigen plus PIKA (Fig 2E). Overall, the results showed that PIKA can help inactivated SARS-CoV vaccine elicit higher neutralizing activity in serum and mucosal secretions.
-
Secretory IgA antibodies in mucosal surfaces can contribute to the protection against viral infection via immune exclusion, intracellular neutralization and immune excretion [17, 44]. The ability to induce a secretory IgA response in mucosal secretions is a very important criterion to evaluate mucosal immunity. For this purpose, all mucosal samples were collected and the SARS-CoV specific secretory IgA antibodies were measured by ELISA. As shown in Fig. 3, SARS-CoV specific sIgA responses could be detected in all mucosal compartments except lung, via intranasal and interperitoneal immunization with antigen and PIKA as an adjuvant. No specific sIgA responses could be detected in intestine and saliva via either intranasal or interperitoneal immunization with antigen alone (Fig. 3A). Intranasal immunization adjuvanted with PIKA, induced significantly higher sIgA titer than interperitoneal immunization with PIKA in intestine and saliva (p < 0.05). Only a low titer of specific sIgA in the vaginal tract could be detected in mice immunized with antigen alone (Fig. 3A), whereas a 500-fold (p=0.002) and 10-fold (p=0.037) increase of sIgA titer could be induced when PIKA was co-administrated with antigen by intranasal and interperitoneal respectively. However, no specific sIgA titer could be detected in lung washing fluid (Fig. 3A) although neutralizing activity could be detected in the lung (Fig. 2D). One concern was how the the lung washing fluid demonstrated detectable neutralizing activity. It is possible that the collection method of lungwashing fluid may interfere with the sample. We therefore tested SARS-CoV specific IgG responses in all samples as well as SARS-CoV specific IgA in serum. Only intranasal immunization adjuvanted with PIKA could induce significant IgA response, whereas intranasal immunization with antigen alone and interperitoneal immunization with or without PIKA induced no detectable IgA (Fig. 3B). Therefore, PIKA showed good adjuvant activity for inactivated SARS-CoV vaccine and enhanced IgA response in mucosal surfaces and serum.
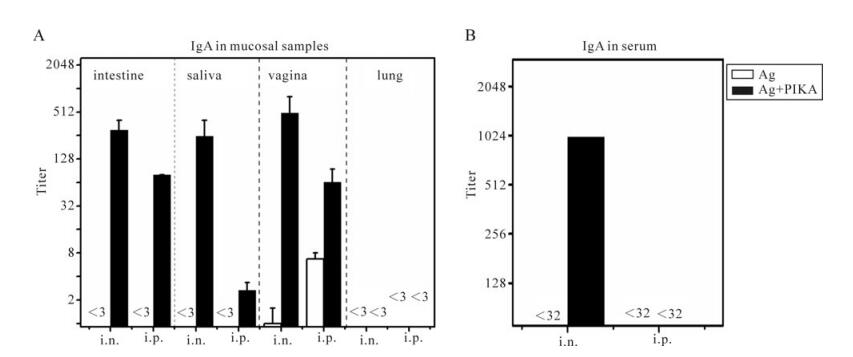
Figure 3. Specific IgA responses in various mucosal samples and serum. Mice were immunized with inactivated SARS-CoV antigen alone or antigen plus PIKA through either intranasally (i.n.) or intraperitoneally (i.p.) route to detect the adjuvanticity of PIKA in inducing specific IgA in mucosal sections and serum. Two weeks after the last immunization, serum (B) and mucosal samples (A) including intestine washing fluid, vagina washing fluid, lung washing fluid and saliva were collected. SARS-CoV-specific IgA titers were determined in diluted samples using ELISA. Each bar represents the arithmetic mean titer of individual group ± SE. The statistical significance of the difference between groups was calculated by using transformed data (Log2) of ELISA mean titers by student's t-test for two groups, or by Tukey's test using ANOVA for three or more groups. Ag, 10µg of inactivated SARS-CoV alone; Ag+PIKA, 10µg of inactivated SARS-CoV plus 100µg of PIKA.
-
the SARS-CoV specific IgG in the pooled mucosal samples as well as in the serum samples The results are summarized in Fig. 4. PIKA could increase a specific IgG response in intestine, saliva, vagina and lung (Fig. 4A, black bar) compared to the respective "antigen only" group (Fig. 4A, black bar) for both intranasal and interperitoneal im-munization routes. The highest IgG titer of 1: 110 000 was induced in the intestine by intraperitoneal immunization with PIKA and IgG alone at a titer of 1: 1 263 was induced without PIKA, whereas a high IgG titer of 1: 39 366 was induced by intranasal immunization with PIKA but a low IgG titer of 1: 8 was induced without PIKA. As for the specific IgG in saliva, a low IgG titer of about 1: 32 could be detected in both the intraperitoneal and the intranasal im-munization groups whereas no discernable titer could be detected in antigen alone groups. In vaginal washes, a specific IgG titer of 1: 1 619 could be induced by intraperitoneal immunization together with PIKA versus a specific IgG titer of 1:18 by immunization without PIKA. Meanwhile, an IgG titer of 1: 10 332 was induced in lung by intraperitoneal immunization with PIKA but an] IgG titer of only 1:423 was induced without PIKA, whereas a lower IgG titer of about 1: 1 500 was induced by intranasal immunization with PIKA but no discernable IgG titer could be detected without PIKA.
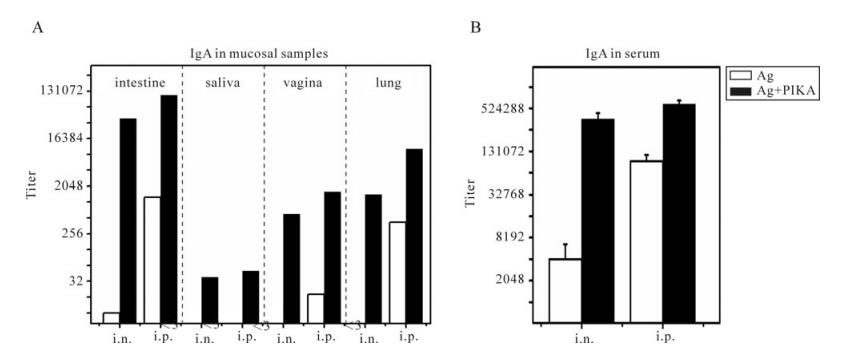
Figure 4. Specific IgG responses in diverse mucosal samples and serum. Mice were immunized with inactivated SARS-CoV antigen alone or antigen plus PIKA through either intranasally (i.n.) or intraperitoneally (i.p.) route to detect the adjuvanticity of PIKA in inducing specific IgG in mucosal sections and serum. Two weeks after the last immunization, serum and mucosal samples including intestine washing fluid, vagina washing fluid, lung washing fluid and saliva were collected. SARS-CoV-specific IgG titers were determined in diluted samples using ELISA (A) specific IgG responses in pooled mucosal samples. Each bar represents the titer of an individual group. (B) specific IgG responses in serum. Each bar represents the arithmetic mean titer of an individual group ± SE. The statistical significance of the difference between groups was calculated by using transformed data (Log2) of ELISA mean titers by student's t-test for two groups, or by Tukey's test using ANOVA for three or more groups. Ag, 10µg of inactivated SARS-CoV alone; Ag+PIKA, 10µg of inactivated SARS-CoV plus 100µg of PIKA.
SARS-CoV specific IgG antibodies in the sera were also tested (Fig. 4B). Intraperitoneal immunization of inactivated SARS-CoV vaccine together with PIKA induced a high IgG titer of 1: 600 000 in serum, which was 5 times the titer induced by immunization by antigen alone (p=0.002). Whereas intranasal immuni-zation of inactivated SARS-CoV vaccine together with PIKA induced significantly higher IgG titers than intranasal immunization with inactivated SARS-CoV antigen alone (p=0.028).
-
To test if serum IgG and mucosal IgA induced by immunization of inactivated SARS-CoV vaccine together with PIKA have SARS-CoV spike protein specificity or not, Western blot assay was performed (Fig. 5). First of all, S', a segment of SARS-CoV S protein (about 60kDa) was identified by traditional Western blot with convalescent serum (diluted 1:4 500) of a SARS patient, (figure 5 lane 1) together with the negative control tested with normal human serum (lane 2). The same S' protein was then used for Western blot assay for serum IgG (diluted 1:500) from the mice intranasally immunized with inactivated SARS-CoV (10µg) plus PIKA. Positive results were obtained and one positive example (serum IgG diluted by 1:500) was presented in lane 3 together with an unimmunized mouse negative control in lane 4. While the same test was performed for intestine; IgA from the mice intranasally immunized with inactivated SARS-CoV (10µg) plus PIKA, a specific band could also be detected by anti-mouse IgA secondary antibody and an example of 1:40 diluted intestine sample was presented in lane 5, with lane 6 for an unimmunized mouse intestine sample as negative control. Therefore there was spike protein specific IgG and IgA induced in serum and in intestine respectively by intranasal immunization of inactivated SARS-CoV vaccine together with PIKA as adjuvant.
Figure 5. Western blot assay of specific serum IgG and intestinal IgA from mice intranasally adjuvanted with PIKA (10µg antigen plus PIKA). Lane 1, Specific serum IgG from SARS patient; 2, Serum IgG of normal human; 3, Specific serum IgG from immunized mice; 4, Serum IgG from mice immunized with PBS; 5, Specific intestinal IgA from immunized mice; 6, Intestinal IgA from mice immunized with PBS.
-
In this study we tested the adjuvanticity of PIKA, a derivative of Poly(I:C), as parenteral and mucosal adjuvant for the induction of humoral immune responses by co-administration with inactivated SARS-CoV vaccine. Our resultssuggest that both intraperitoneal and intranasal administration of inactivated SARS-CoV vaccine together with PIKA could induce significantly higher neutralizing activity, specific IgA and IgG responses in systemic and mucosal sites. PIKA was even more effective as an adjuvant than CpG in inducing antibody responses for inactivated SARS-CoV vaccine (data not shown). Although intraperitoneal immunization of inactivated SARS-CoV vaccine alone could induce a certain level of neutralizing activity in serum as well as in mucosal sites (Fig. 2), co-administration of inactivated SARS-CoV vaccine with PIKA as adjuvant could induce much higher neutralizing activity. Furthermore, when an intranasal immunization was adopted, PIKA was obligatorily needed for inducing neutralizing activity (Fig. 2). Some other studies showed similar phenomena when alum, IL-1, IL-12 and GM-CSF were used as adjuvants and delivered through the parenteral route; this approach could help to elicit HIV immunogens specific IgA and IgG responses in vaginal, fecal and saliva samples [4]. A parenterally administered virus-like particle even elicited 90% to 100% efficacy against infection and pre-invasive disease in previously uninfected individuals [15].
For determining correlation between neutralizing activity and antibody in serum and mucosal secretion, both IgG and IgA responses in serum and in mucosal secretion were tested. In our study, neutralizing activity was detected in lung samples from three groups of mice, in which only specific IgG but not IgA could be detected (Fig. 2-4). Antibody analysis also indicated that it was specific IgG but not specific IgA response at mucosal sites that was correlated with neutralizing activity. It is very possible that mucosal IgG could also play an important role in contributing to the neutralizing activity at mucosal sites. It has been propose [41], serum IgG induced in parenteral immunization could find its way to the mucosae to exert protective effects. The relatively high con-centrations of IgG seen at particular mucosal sites may reflect a combination of constitutive transcellular or paracellular transport from the systemic compartment and specific transport mechanism [41]. Therefore, detection of IgG response in mucosal sites is also useful for evaluation of mucosal immune response and related neutralizing activity.
Because SARS-CoV infection occurs at mucosal surfaces, such as lung, digestive surfaces and even vaginal surface [20, 21, 26, 38, 40], the induction of a neutralizing activity which could block virus attachment and infection is more important in SARS-CoV prevention. Our study showed that PIKA was effective at inducing neutralizing activity at mucosal sites when co-administered by intraperitoneal and intranasal immunization (Fig. 2). Intranasal immuni-zation appeared less effective than intraperitoneal immunization at eliciting neutralizing activity, even when adjuvanted with PIKA. This may partially result from certain loss of vaccine antigen, dilution by mucosal secretion, capture by mucus gels, attack by proteases and nucleases, or exclusion by epithelial cells when delivered through mucosal routes [30]. However, it should be noted that intranasal immunization could induce much higher specific IgA responses than intraperitoneal immunization. Many studies already demonstrated that mucosal sIgA can inhibit viral infection via immune exclusion, intra-cellular virus neutralization, and transepithelial transport of immune complexes [17, 28, 44]. Even rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity can protect mice against infection by] rotavirus via an intracellular neutralization mechanism [5]. SARS-CoV-specific sIgA may also provide a similar kind of protection against SARS-CoV invasion at mucosal sites in the absence of classical neutralizing activities. If this is true, the in vitro neutralizing activity may only reflect partial correlation with the protection effect of immune response.
The adjuvanticity of PIKA may be mostly derived from its main component Poly(I:C). However, the adjuvant mechanism of Poly(I:C) is not clearly understood yet although it is believed to be related with to dsRNA structure and TLR3 mediated immune response. In-vivo, the presence of viral dsRNA can trigger cellular immune responses to viral infection through the activation of dsRNA-dependent enzymes including PKR, and the IFN-inducible 2'-5'-adenylate synthase/Rnase L system [12, 16]. In addition, mammalian TLR3's can recognize viral dsRNA, leading to the activation of NF-κB and the production of type 1 interferons which can serve as a bridge between the innate and adaptive immune systems [1]. Takeshi Ichinohe et al. demonstrated that intranasal immuni-zation of influenza HA vaccine adjuvanted with Poly(I:C) could enhance the expression of TLR3 in nasal associated lymphoid tissues (NALTs) in mice and elicited effective protection against influenza virus infection [11].
For development and application of an adjuvant for human use, the priority is its safety. CT is the most potent known mucosal adjuvant, but it has adverse side effects, such as nasal discharge in humans. Here we determined the mucosal adjuvanticity of a new adjuvant, PIKA, which is a derivative of Poly(I:C). Poly(I:C) itself is toxic to human and can be rapidly hydrolyzed despite its strong adjuvanticity [6]. PIKA is an improved adjuvant which was formulated as a complex of Poly(I:C), kanamycin and calcium chloride. Being a positively charged molecule, kanamycin is able to neutralize the negative charge of Poly(I:C), rendering Poly(I:C) more stable and resistant to hydrolysis. The calcium chloride facilitates entry of Poly(I:C) into cells [26]. All together, PIKA is
In summary, we have demonstrated that both intraperitoneal and intranasal administration of PIKA as an adjuvant can significantly raise the humoral immune response with neutralizing activity against SARS-CoV in serum and mucosal sites. Therefore, PIKA is a good adjuvant candidate and can be also used as mucosal vaccine.














 DownLoad:
DownLoad: