HTML
-
Wheat, the third most-produced cereal crop in the world, is often infected with viruses, resulting in significant losses in grain yield and poor quality. Among the known wheat infecting viruses, wheat dwarf virus (WDV) is considered to be an important pathogen to wheat (Lindblad and Sigvald 2004). Since the first report of WDV infection in Czech, this virus has now been identified in crops in Europe, Asia and Africa (Vacke 1961; Kapooria and Ndunguru 2004; Lemmetty and Huusela-Veistola 2005; Xie et al. 2007; Ekzayez et al. 2010).
As a member of the genus Mastrevirus in the family Geminiviridae, WDV possesses a single-stranded circular DNA genome, which encodes four proteins. The viral sense strand, which consists of two open reading frames (ORFs), encodes the coat protein (CP) and the movement protein (MP). The complementary strand encodes replication associated protein (Rep) and replication protein A (RepA) (Schalk et al. 1989). WDV can infect multiple members in the family Poaceae, including wheat, barley, and grasses (Avena fatua and Poa pratensis) (Ramsell et al. 2008). According to the host susceptibility and DNA sequencing, WDV was divided into two different stains (i.e. the wheat strain and the barley strain) (Wu et al. 2015). Studies indicated that WDV can be transmitted by leafhopper (Psammotettix alienus) at both nymph and adult stages in a persistent, nonpropagative manner in fields (Lindblad and Sigvald 2004).
Due to the absence of WDV resistant wheat varieties, the current WDV control method relies mostly on vector control using pesticides. Thus, accurate, sensitive and easyto-use WDV detection methods become crucial for the effective control of WDV. To date, several WDV detection methods, including a squash blot (Bendahmane 1995), PCR or Real-time PCR (Achon et al. 2006; Koklu et al. 2007; Zhang et al. 2010), nucleic acid spot hybridization (NASH) (Jin et al. 2015), and a polyclonal antibody (PAb)-based enzyme-linked immunosorbent assay (ELISA) (Nygren et al. 2015), have been reported. Compared with PCR, realtime PCR and nucleic acid spot hybridization, serological assays were considered to be more convenient assays for large-scale epidemiological studies and on-site detection of viruses during field surveys (Li et al. 2015; Liu et al. 2016; Chen et al. 2017). It is generally accepted that a reliable serological detection assay depends largely on the specificity and sensitivity of the antibody used in the assay.
In this study, four monoclonal antibodies (MAbs) specific for WDV were produced using a purified recombinant WDV CP as the immunogen. Using these prepared MAbs, an antigen-coated-plate enzyme-linked immunosorbent assay (ACP-ELISA) and a dot-ELISA for WDV detection were developed. Results of field sample tests using these two new methods showed that WDV can be readily detected in the WDV-infected leaf crude extracts and the virus is now prevalent in wheat crops in the Shaanxi and Qinghai provinces, China. These MAbs and the serological assays can be very useful for WDV diagnosis, forecast, and management during wheat production.
-
WDV isolates were identified in wheat plants showing virus-like symptoms in 2014 in the Shaanxi Province, China as described previously (Zhou et al. 2003). PAV, GPV and GAV strain of barley yellow dwarf virus (i.e. BYDV PAV, BYDV GPV and BYDV GAV), wheat yellow mosaic virus (WYMV), barley yellow mosaic virus (BaYMV) and Chinese wheat mosaic virus (CWMV) were isolated from wheat fields in China and characterized by RT-PCR followed by nucleotide sequencing. The isolated viruses were maintained individually in authors' laboratory. For a field survey conducted in this study, a total of 128 wheat samples showing virus-like symptoms were collected from wheat fields in the Shaanxi and Qinghai provinces, China, during the 2014–2016 growing seasons and stored at -80 ℃ till use.
-
Total DNA was extracted from a WDV-infected wheat sample with the CTAB method as described (Gawel and Jarret 1991). The WDV CP gene was then PCR-amplified from the extracted total DNA using the primer set designed according to the published WDV sequence [GenBank Accession No. JQ836568, WDV-CP-F (5'-ACGGATCCAT GGTGACCAACAAGGACTCCCGA-3') and WDV-CP-R (5'-CTAAGCTTTTATTGAATCCCAATGGATTTGA-3'), the underlined sequences were a BamH Ⅰ and a Hind Ⅲ restriction enzyme sites)]. The amplified PCR fragments were digested with BamH Ⅰ and Hind Ⅲ restriction enzymes, and cloned into the His-tagged prokaryotic expression vector, pET-32a. Sequence and orientation of the cloned CP gene in the pET-32a vector was confirmed by DNA sequencing. A correct pET-32a-CP vector was transformed into Escherichia coli BL21 (DE3) cells. His-tagged WDV CP recombinant protein was expressed, purified and analyzed as described by Liu et al. (2016).
-
Eight-week-old BALB/c mice were used to produce MAbs specific for WDV CP. The purified recombinant WDV CP with or without Freund's adjuvant was injected intraperitoneally into BALB/c mice as described previously (Chen et al. 2017). After the forth immunization, spleen cells were obtained from the immunized mice and used to produce hybridoma as described (Liu et al. 2016). Screening of hybridomas secreting anti-WDV MAbs and production of ascitic fluids were as described by Li et al. (2015). Specificity of the resulting MAbs was determined by Western blot as described previously (Wu et al. 2013).
-
Phalanx tests were performed to determine the working dilutions of MAbs and alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) for ACP-ELISA as described previously (Shang et al. 2011; Wu et al. 2013) with specific modifications. Briefly, wheat leaf samples were ground in liquid nitrogen and then homogenized in 0.05 mol/L sodium bicarbonate buffer, pH 9.6, at a ratio of 1 g tissue in 20 mL buffer. The homogenized samples were centrifuged at 5000 ×g for 3 min and the supernatants were collected for further use. Wells of 96-well microtiter plates were coated with the supernatant from a healthy wheat plant (negative control) or from a WDV-, WYMV-, BYDV PAV-, BYDV GAV-, BYDV GPV-, BaYMV-, or CWMV-infected wheat plant (100 lL supernatant/well). After overnight incubation at 4 ℃, the plates were rinsed three times with 0.01 mol/L phosphate buffered saline (PBS) containing 0.05% Tween-20 (PBST, pH 7.4). The wells were then blocked with 250 lL 3% dried skimmed milk in a 0.01 mol/L PBS for 30 min at 37 ℃. Diluted anti-WDV MAb solution (100 lL) was added into each well and the plates were incubated at 37 ℃ for 1 h. After three rinses with PBST, a diluted AP-conjugated goat anti-mouse IgG solution (100 lL) was added into each well and the plates were incubated at 37 ℃ for 1 h. After four rinses with PBST, p-nitrophenyl phosphate substrate solution was added into each well and the plates were incubated at 37 ℃ for 30 min. The OD405 absorbance value of individual well was measured with a microplate reader.
The dot-ELISA was carried out as described by Wu et al. (2014). Leaf crude extract from a healthy or a WDVinfected wheat plant was used as a negative or a positive control during the assays. The dots representing positive plant extracts developed a purple color within 10–20 min post the addition of the substrate solution.
-
Detection of WDV infection in field-collected wheat samples was performed by PCR using the WDV-CP-F and WDV-CP-R primer set described above. The resulting PCR products were cloned individually into the pMD18-T vector (TaKaRa Biotechnology, Dalian, China) and sequenced individually by the Invitrogen Shanghai Sequencing Department (Shanghai, China) followed by sequence analyses through blast searching the data base available at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov).
-
To investigate the spread of WDV in wheat fields, we collected 128 wheat plant samples from wheat fields in the Shaanxi and Qinghai provinces, China, during the 2014–2016 growing seasons and tested them individually for WDV infection using the two serological methods developed in this study, and PCR followed by nucleotide sequencing.
Sources of Viruses and Field Samples
Prokaryotic Expression of WDV CP
Preparation of MAbs
ACP-ELISA and Dot-ELISA
PCR Detection of WDV and Nucleotide Sequencing
Field Survey for WDV Infection
-
The full-length WDV CP gene sequence with 783 nucleotides was PCR-amplified. After double digestion with BamH Ⅰ and Hind Ⅲ restriction enzymes, the PCR fragment was inserted into the expression vector pET-32a to produce pET-32a-CP. DNA sequencing was performed to confirm the CP gene nucleotide sequence and orientation. A correct recombinant plasmid was transformed into Escherichia coli BL21 (DE3) cells to express recombinant WDV CP. After IPTG induction, the E. coli BL21 (DE3) cells harboring the pET-32a-CP vector accumulated a 50 kDa fusion protein (Fig. 1A). E. coli BL21 (DE3) cells transformed with the parental pET-32a vector produced an approximately 20 kDa protein, similar to the molecular mass of the thioredoxin-tag. The non-denatured recombinant CP fusion protein was purified using the Ni–NTA agarose method (Qiagen, MD, USA) as described previously (Liu et al. 2017). The expressed recombinant WDV CP protein was later confirmed by Western blot using an anti-His tag MAb (Fig. 1B).
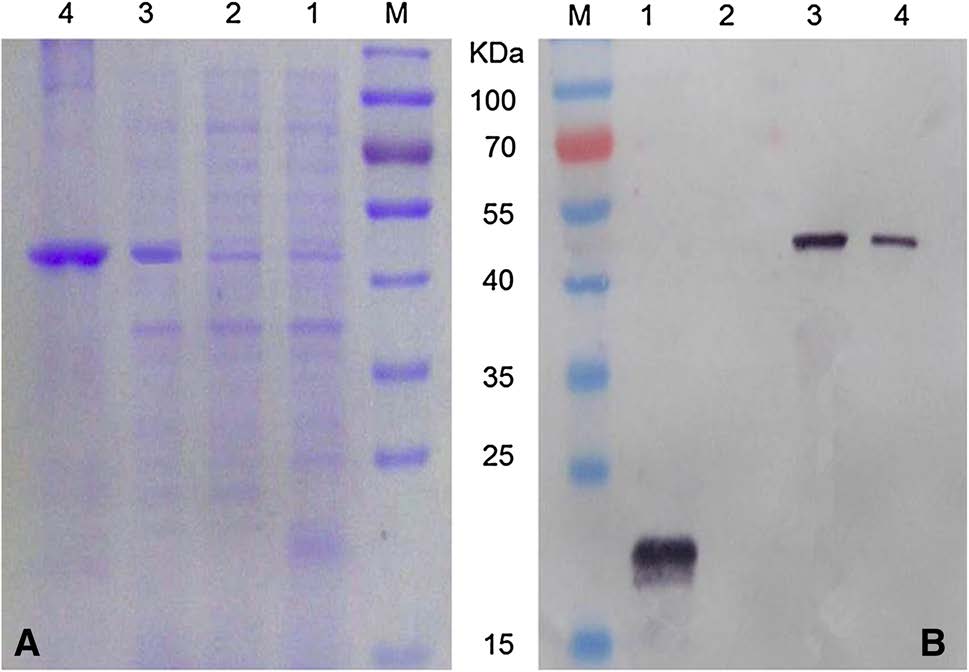
Figure 1. SDS-PAGE (A) and Western blot (B) analyses of the recombinant WDV CP protein. Lane M, protein molecular weight marker. Lanes 1 and 2, E. coli BL21 (DE3) harboring pET-32a induced with and without 0.5 mmol/L IPTG. Lane 3, E. coli BL21 (DE3) harboring pET-32a-CP induced with 0.5 mmol/L IPTG. Lane 4, Purified recombinant WDV CP.
-
BALB/c mice were immunized with purified recombinant WDV CP. After the fourth immunization, four hybridoma lines (18G10, 9G4, 23F4 and 22A10) secreting anti-WDV CP MAbs were obtained through four time cell fusions, antibody specificity and sensitivity analyses, and cell limiting dilution cloning. Ascitic fluids with MAbs were produced by intraperitoneal inoculations of hybridoma cells to pristane-primed BALB/c mice. IgG of WDV specific MAb was precipitated from different ascitic fluids with saturated ammonium sulfate. Isotypes of the four MAbs were determined to be IgG1, κ light chain. Yield of IgG in ascites was determined at 5.87 to 10.14 mg/mL, and the titers of the four MAbs ranged from 10-6 to 10-7 as determined by an indirect ELISA (Table 1).

Table 1. Properties of the obtained anti-WDV monoclonal antibodies.
Western blot was then used to determine the specificity of the anti-WDV MAbs. Results of the assays indicated that the four MAbs reacted strongly and specifically with approximately 30 kDa WDV CP in the WDV-infected wheat samples as well as the 50 kDa recombinant WDV CP fusion protein (Fig. 2). As expected, no visible protein bands were seen in the lane loaded with an extract from a healthy wheat plant (Fig. 2).

Figure 2. Specificity analyses of anti-WDV MAbs by Western blot. All the SDS-PAGE gels had the same protein loadings but were probed with different MAbs. Lane 1, protein from a healthy wheat plant. Lane 2, protein from a WDV-infected wheat plant. Lane 3, purified recombinant WDV CP fusion protein. Lane M, protein molecular markers. Names of the MAbs are indicated below the figures.
-
The optimal working dilutions of MAbs and the AP-conjugated goat anti-mouse IgG for the ACP-ELISA were determined by the phalanx tests. Results of the phalanx tests indicated that WDV could be reliably detected in crude extracts from WDV-infected wheat plant tissues using MAb 22A10, 23F4, 18G10 or 9G4, diluted at 1:6, 000, 1:6, 000, 1:5, 000 and 1:5, 000 (v/v). The optimal dilution of AP-conjugated goat anti-mouse IgG was determined at 1:8, 000 (v/v). Using the optimal working dilutions described above, an ACP-ELISA for WDV detection was developed.
The specificity assay using the developed ACP-ELISA protocol demonstrated that WDV could be reliably detected in the WDV-infected wheat samples but it had a negative reaction with WYMV-, BYDV PAV-, BYDV GAV-, BYDV GPV-, BaYMV-or CWMV-infected, or the healthy wheat sample (Fig. 3A).
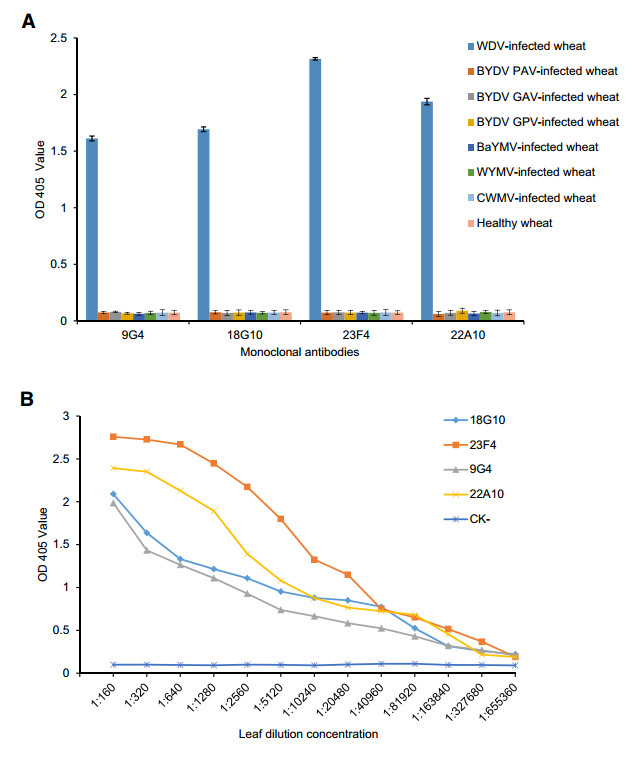
Figure 3. Specificity (A) and sensitivity (B) analyses of the developed ACP-ELISA using anti-WDV MAbs. CK-, an extract from a healthy wheat plant sample.
Sensitivity assay showed that the developed ACPELISA methods based on MAb 23F4, 22A10, 9G4 or 18G10, could detect the virus in WDV-infected wheat plant crude extracts diluted at 1:163, 840, 1:163, 840, 1:81, 920 or 1:81, 920 (w/v, g/mL), respectively (Fig. 3B). These results showed that the newly established ACP-ELISA is a highly sensitive and specific method for detection of WDV in wheat samples.
-
Phalanx tests were also performed to determine the optimal working dilutions of the four MAbs and the AP-conjugated goat anti-mouse IgG for dot-ELISA. Results of the tests showed that the optimal working dilutions for MAb 23F4, 22A10, 9G4 and 18G10 were all 1:5, 000 (v/v). For the APconjugated goat anti-mouse IgG, the optimal working dilution was found to be 1:8, 000 (v/v). Using this newly developed dot-ELISA, WDV was reliably detected in the dot of crude extract from a WDV-infected wheat plant or in the dot of purified recombinant WDV CP (Fig. 4, Column 1 and 2). No detection signal was observed in the dots of crude extracts from a WYMV-, BYDV PAV-, BYDV GAV-, BYDV GPV-, BaYMV-or CWMV-infected wheat plant (Fig. 4A, Column 3–8) or from a healthy wheat plant (Fig. 4A, Column 9). Further assays indicated that dotELISAs based on MAb 23F4, 22A10, 18G10 or 9G4, could be used to detect the virus in WDV-infected wheat plant crude extracts diluted at 1:5, 120, 1:1, 280, 1:1, 280 and 1:640 (w/v, g/mL), respectively (Fig. 4B).

Figure 4. Specificity (A) and sensitivity (B) analyses of the developed dot-ELISA using anti-WDV MAbs. A Specificity test of the dotELISA. Columns 1 and 2 have dots of an extract from a WDVinfected wheat plant sample and from the purified recombinant WDV CP, respectively. Columns 3–8 have dots of an extract from a WYMV-, CWMV-, BYDV GAV-, BYDV GPV-, BYDV PAV-or BaYMV-infected wheat plant sample. Column 9 has the dot of an extract from a healthy wheat plant. Each treatment has two dots. MAbs used for the treatments are indicated on the left side. B Sensitivity test of the dot-ELISA. Dots in each panel represent a WDV-infected or a healthy wheat plant. Dilutions of the wheat plant extracts are indicated on the top of the figures and the names of the MAbs are indicated on the right side.
-
According to the results from the sensitivity and specificity assays, MAb 23F4 was selected to detect WDV in fieldcollected wheat samples through ACP-ELISA and dotELISA. To further confirm the detection results, all the samples were retested by PCR using WDV specific primers.
For this study, a total of 128 wheat plant samples were collected from wheat fields in Hancheng city of Shaanxi Province, and Xining city of Qinghai Province, and tested for WDV infection. Results of the two serological assays indicated that 97 of the 128 samples were tested positive for WDV infection (Fig. 5). Further validation using PCR was agreed with the results obtained by the two serological detection methods (Fig. 5). Sequencing of the resulting PCR products followed by sequence alignment showed that the WDV isolates detected in the field samples sheared 96.5%–97.7% sequence similarity with the published wheat dwarf virus isolate SXHC-2 sequence (GenBank Accession No. JQ836568). Taken together, we have shown that the newly developed ACP-ELISA and dot-ELISA methods using MAb 23F4 is highly sensitive and accurate for detection of WDV in field-collected wheat plant samples.
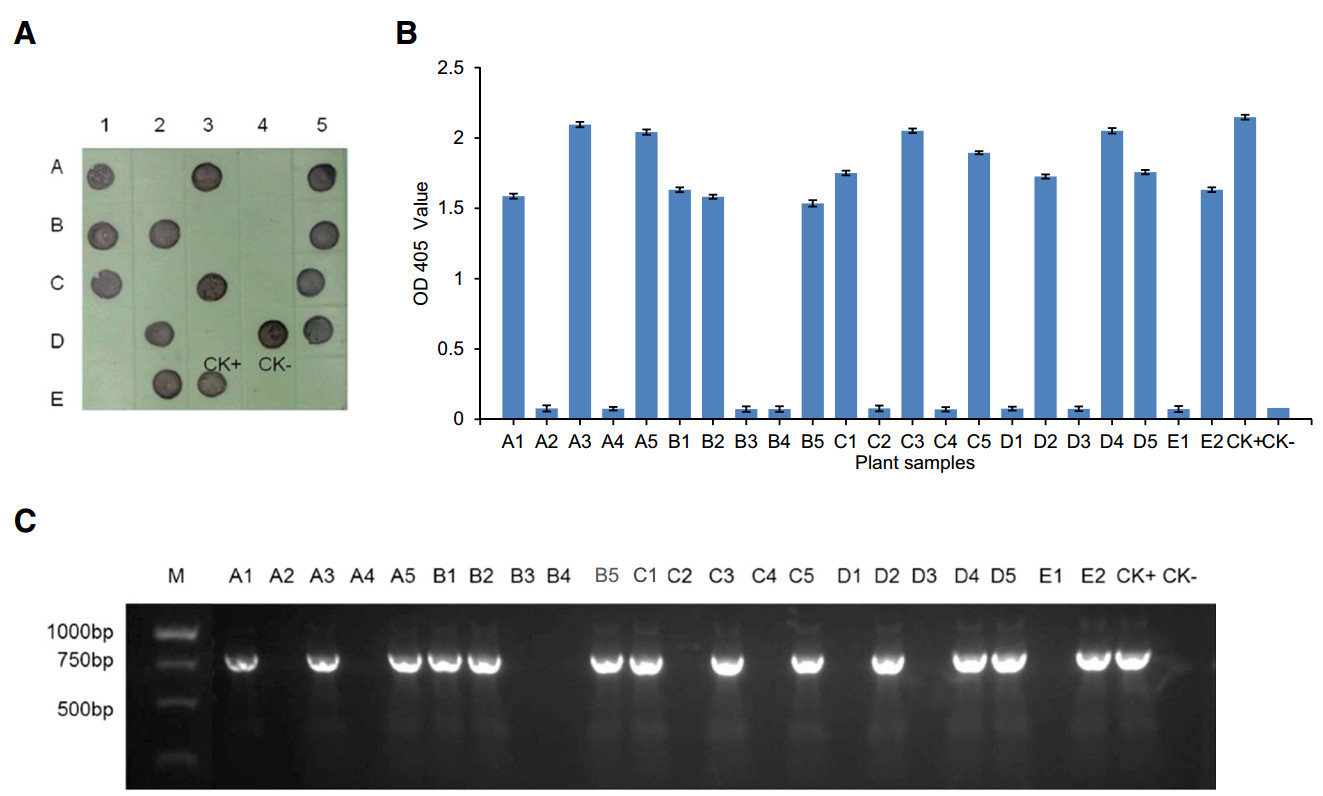
Figure 5. Detection of WDV in field-collected wheat samples by dotELISA, ACP-ELISA and PCR. A Detection of WDV in fieldcollected wheat samples by dot-ELISA. A representative blot was showing sample detection result. The dots labeled CK + and CKrepresent a WDV-infected and a healthy wheat control sample, respectively. Purple colored dots indicate WDV-infected wheat samples. The same samples used in (A) were tested again by ACPELISA (B) and PCR (C).
Prokaryotic Expression and Purification of WDV CP
Production and Characterization of MAbs against WDV CP
ACP-ELISA Detection of WDV
Dot-ELISA for WDV Detection
Detection of WDV in Field-Collected Wheat Samples
-
Wheat crop worldwide is often infected with various viruses. Among the viruses reported for wheat, WDV is known to be present worldwide and often causes serious wheat yield losses (Lindblad and Sigvald 2004). To prevent or minimize the damages caused by WDV, development of a rapid, accurate and high-throughput detection technology for this virus is crucial. This technology can also benefit epidemiological studies in different regions and breeding work for WDV resistant varieties.
Several WDV detection methods are currently available. For example, PCR and Real-time PCR were reported for WDV detection by multiple research groups worldwide (Achon et al. 2006; Koklu et al. 2007; Zhang et al. 2010; Gadiou et al. 2012; Wang et al. 2016). Immunolabeling using a polyclonal antibody (PAb) against WDV CP showed that the virus was accumulated in the anterior and in the middle part of midgut in its leafhopper vector (Wang et al. 2014). Although PCR-based detection technique is highly sensitive and accurate for WDV detection, it is less cost effective but labor intensive for large-scale field studies. In contrast, serological assays for plant virus detection are simple to use, specific and sensitive. Consequently, serological assays are considered to be suitable for high-throughput field surveys by many laboratories and farm advisers. For example, recent reports have indicated that ACP-ELISA, DAS-ELISA, tissue print-ELISA and dot-ELISA using virus specific MAbs can be applied to detect rice black-streaked dwarf virus (RBSDV), rice ragged stunt virus (RRSV), zucchini yellow mosaic virus (ZYMV) and several other economically important plant viruses accurately and efficiently in both plant tissues and their specific insect vectors (Wu et al. 2013; Liu et al. 2014; Chen et al. 2017).
In this paper, we reported the production of four hybridoma cell lines (18G10, 9G4, 23F4 and 22A10) secreting specific WDV MAbs through the conventional hybridoma technique. Using these specific MAbs, we have developed an ACP-ELISA and a dot-ELISA for detecting WDV in wheat plant tissue samples. Our results showed that the ACP-ELISA and dot-ELISA based on MAb 23F4 can detect the virus in WDV-infected wheat plant tissue crude extracts diluted at 1:163, 840 and 1:5, 120 (w/v, g/mL), respectively. To our knowledge, these two methods are currently the most sensitive and specific serological methods for WDV detection. The accuracy of the two serological assays was confirmed by PCR during the study using field-collected wheat plant samples. Based on the survey results using the collected field samples, we concluded that WDV is now widely present in the wheat growing regions in the Shaanxi and Qinghai provinces, China. We consider that the ACP-ELISA and dot-ELISA developed in this study can be of valuable for forecasting WDV occurrence in fields and establishment of effective management strategy for wheat dwarf disease.
-
We are grateful to Dr. Xinshun Ding (Samuel Roberts Noble Foundation, Ardmore, USA) for his valuable comments and manuscript edits. This work was supported by Public Science and Technology Research Funds Projects of Agriculture (201303021), and the National Basic Research Program (973) of China (No. 2014CB138400).
-
prepared the MAbs and carried out the immunoassays. RC performed the PCR detection of wheat samples. XPZ and JXW conceived of the study, participated in its design and helped to draft the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
The animal experiments were performed in accordance with the Principles of the Helsinki accords and approved by the Animal Experimentation Ethics Committee of Zhejiang University, Hangzhou, China.















 DownLoad:
DownLoad: