-
Dear Editor,
Mink circovirus (MiCV), which is clustered in the genus Circovirus of the family Circoviridae, was first described in minks from farms in Dalian, China in 2013 (Lian et al. 2014). The complete single-stranded circular genome of the virus is 1, 753 nucleotides long and contains two major open reading frames (ORFs), designated ORF1 (Rep gene) and ORF2 (Cap gene) (Lian et al. 2014; Ge et al. 2018). Sequence analysis has shown that MiCV is most closely related to mammalian circoviruses, such as bat circovirus (BatCV), porcine circovirus (PCV), and dog circovirus (dogCV) (Ge et al. 2018). Epidemiological investigations have revealed that the mink circovirus is very prevalent in China, with a positive rate of up to 54.6% (101/185) on some mink farms in China (Lian et al. 2014; Wang et al. 2015; Ge et al. 2018). The virus is found in the liver, digestive tract, and fecal specimens of minks, with diarrhea as the main clinical sign (Lian et al. 2014).
Minks, foxes and raccoon dogs are important fur-bearing animals raised in China. However, since the early 1990s, more than 10% of minks have exhibited lethargy, anorexia, diarrhea, growth retardation and low body weight in the fall on many mink farms in China. The feces of the diseased animals are initially white and red and then become a variety of colors. Several days after diarrhea occurs, more than 30% of diseased minks die, and the fur of recovered minks is unkempt and of reduced quality. Recently, some foxes and raccoon dogs on farms in China have exhibited similar clinical signs, such as lethargy, anorexia, pale muzzles, and unkempt fur. To date, the exact reason is still unknown. Because the clinical signs of these diseased minks, foxes and raccoon dogs are very similar to those reported by Lian et al. (2014), we hypothesized that the animals may be infected with mink circovirus (MiCV).
Circovirus infections may play a role in autoimmunity by changing the homeostatic balance of proinflammatory cytokines and the immune system, indirectly affecting the severity of diseases caused by other pathogens (Opriessnig and Halbur 2012; Shulman and Davidson 2017). Previous studies have reported that concurrent infection of circovirus and other pathogens can result in serious immunosuppression and secondary infection (Gillespie et al. 2009; Yi and Liu 2010; Zhai et al. 2011; Opriessnig and Halbur 2012; Meng 2013; Sun et al. 2015; Yang et al. 2015; Ren et al. 2016; Hu et al. 2017). To date, little is known about natural MiCV infection in foxes and raccoon dogs and about coinfection with other pathogens. Therefore, the present study intends to investigate MiCV infection in minks, foxes and raccoon dogs in China as well as the coinfection of MiCV with Escherichia coli (E. coli) and mink enteritis parvovirus (MEV), which are major pathogens of minks.
In the autumn and winter of 2017, large-scale diarrhea, which was called "autumn diarrhea" by local farmers, occurred in fur-bearing animals, including minks, foxes and raccoon dogs, in China. The diseased animals were mainly newborn animals; the number of sick old animals was relatively small, and the symptoms were relatively mild or no disease occurred in the old animals. Diarrhea was the typical symptom of this disease, with hoary feces in the early stage and bloody stools and black feces occurring later. Ultimately, the diseased animals ceased eating and died 3 or 5 days later. Antibiotic treatment was useless for the diseased animals (data not shown).
A total of 80 samples of minks, foxes and raccoon dogs were collected from 21 farms during September and December of 2017, including one farm in the Shandong province, five in the Liaoning province, seven in the Hebei province, five in the Heilongjiang province, and three in the Jilin province (Supplementary Table S1).
Necropsy was performed on the carcasses, and the lungs, liver, spleen, kidneys and mesenteric lymph nodes of the diseased animals were collected. Histologic results showed that the main pathologies of the diseased animals were enlarged mesenteric lymph nodes and visceral hemorrhages, including those of the gastrointestinal mucosa, liver, spleen and kidney, followed by degenerative necrosis (Supplementary Figure S1). These results demonstrated that the typical necropsy symptoms of circovirus infection can be observed in dead minks, foxes and raccoon dogs.
In order to confirm the MiCV infection, lymph nodes of the diseased animals were ground, homogenized as previously described (Cha et al. 2014), and examined using transmission electron microscopy. Electron microscopy (EM) analysis of the negatively stained virion pellets was carried with a JEM 1200 EX Ⅱ transmission electron microscope (JEOL USA, Peabody, MA, USA). The results showed that there were many typical circovirus particles, with diameters of approximately 20 nm, in the lymph nodes of the diseased animals (Fig. 1A).
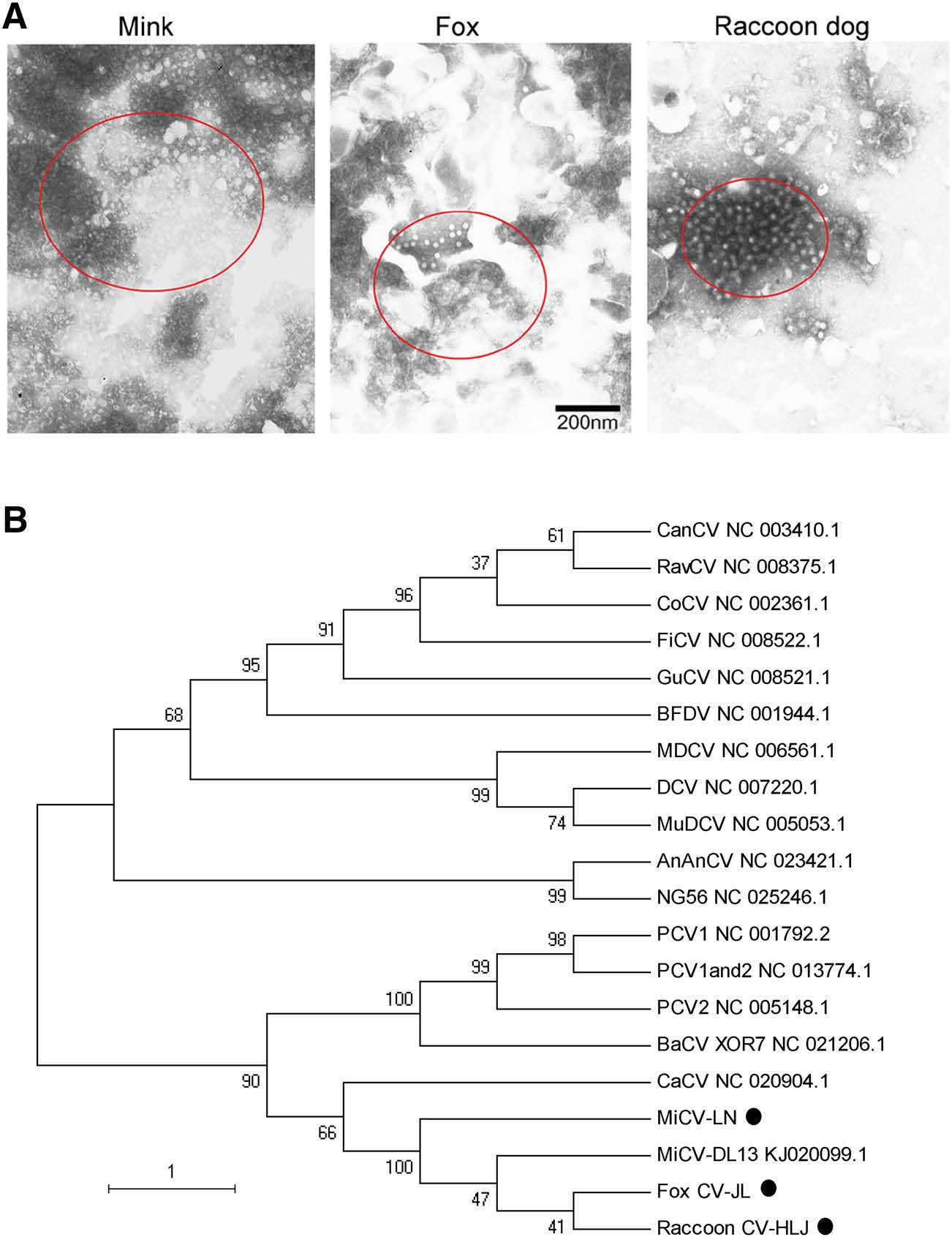
Figure 1. Mink circovirus can infect minks, foxes and raccoon dogs. (A) Transmission electron microscopy (TEM) ultrastructural analysis of homogenized lymph nodes from the diseased animals. The circles indicate the virus particles 20 nm in diameter observed by TEM. The scale bar indicates 200 nm. (B) Phylogenetic tree of MiCVs isolated in this study and other circoviruses. A phylogenetic tree was constructed based on the Rep gene of MiCV using the neighborjoining method in MEGA 5.1. Bootstrap analysis with 1000 replicates was applied to assess the reliability of the reconstructed phylogenies. The evolutionary distances were computed using the maximum composite likelihood method and are presented as the number of base substitutions per site. The scale bar indicates the estimated evolutionary distance. AnAnCV, Anguilla anguilla circovirus; BaCV, bat circovirus; BFDV, beak and feather disease virus; CaCV: circovirus in tissues of dogs with vasculitis and hemorrhage; CanCV, canary circovirus; CoCV, columbid circovirus; DCV, duck circovirus; FiCV, finch circovirus; GuCV, gull circovirus; MDCV, Muscovy duck circovirus; MiCV-DL13, mink circovirus; MuDCV, Mulard duck circovirus; NG56, Silurus glanis circovirus; PCV1, porcine circovirus type 1; PCV2, porcine circovirus type 2; PCV1 and 2, porcine circovirus type 1/2a; RavCV, raven circovirus. The new isolated strains were highlighted with dots
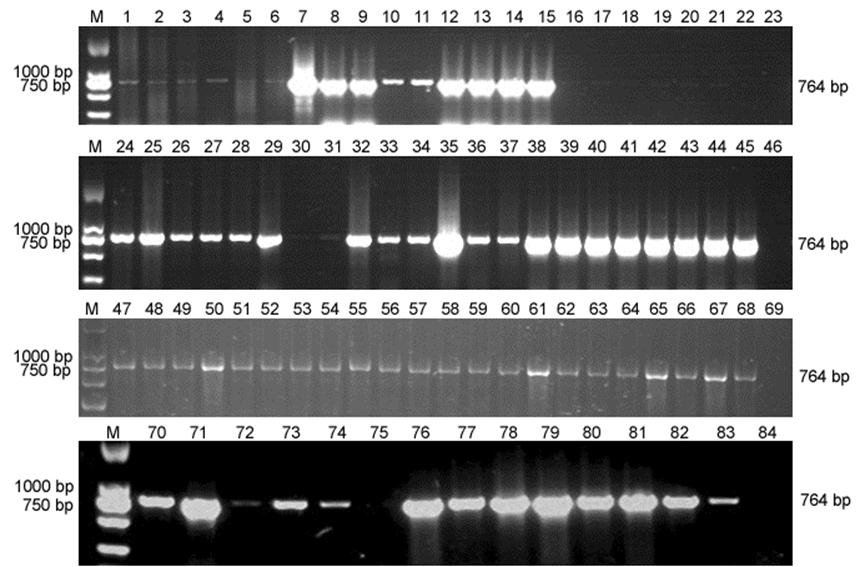
To evaluate the infection of MiCV in the collected samples, total viral DNA was extracted from the prepared supernatants using a Universal Genomic DNA Extraction Kit (Takara Bio, Inc., Dalian, China). MiCV-specific primers(CV-F1:50-TTCCTGACGGAGGCTAACTGTGTGT- 30;CV-R1:50-TCCCCCCCTTGTCTCATAACTTGGT-30) were designed and synthesized according to the highly conserved Rep gene sequence of MiCV-DL13 (GenBank accession number: KJ020099). Using MiCV-specific PCR, MiCV was detected in 78 of 80 (78/80, 97.5%) samples, with only one mink (lane 22 in Supplementary Fig. S2) and one raccoon dog (lane 75 in Supplementary Fig. S2) testing negative for MiCV. These results indicated that the rates of MiCV infection in foxes, minks and raccoon dogs were very high on some fur farms in China. Furthermore, the results in Table 1 also showed that the most sensitive animal to the virus was the fox, followed by the mink and the raccoon dog.

Table 1. Infection patterns of mink circovirus (MiCV) in this study
Subsequently, three PCRfragmentsfrom the virus inminks, foxes and raccoon dogs were sequenced and designated as MiCV-LN (GenBank accession number: MH323414), Fox CV-JL (GenBank accession number: MH323415), and Raccoon CV-HLJ (GenBank accession number: MH323416), respectively. To explore the relationship among the isolates and other circoviruses, phylogenetic tree was constructed based on the Rep gene of the threefragments and the sequences reported in GenBank using the neighbor-joining method in MEGA 5.1. The results showed that MiCV-LN, Fox CV-JL, and Raccoon CV-HLJ were grouped into one subgroup with MiCV-DL13 (GenBank accession number: KJ020099.1) reported by Lian et al. (2014) (Fig. 1B). The sequence comparison showed that MiCV-LN, Fox CV-JL, and Raccoon CVHLJ had 99% homology with MiCV-DL13.
Furthermore, we analyzed the occurrence of coinfection of MiCV with E. coli and MEV. E. coli were isolated and identified with a bacterial biochemical test instrument according to the instruction manual (VITEK® 2 Compact, bioMe′rieux, France). MEV was detected using PCR according to the protocols described previously (Zhang et al. 2007). The results demonstrated that singular infections with MiCV were found in 70 (89.7%) of the 78 samples, while coinfection of MiCV and E. coli was found in 9% (7/78) and triple infections (MiCV + E. coli + MEV) were found in 1.3% (1/78) of the samples (Table 1). For each species, singular infection with MiCV in minks, foxes, and raccoon dogs occurred in 93.0% (40/43), 95.5% (21/22) and 69.2% (9/13) of the animals, respectively. Coinfection of MiCV and E. coli in minks, foxes, and raccoon dogs occurred in 7.0% (3/43), 4.5% (1/22) and 23.1% (3/13) of the animals, respectively. Meanwhile, one raccoon dog was coinfected with MiCV, E. coli and MEV, and the positive rate was 1.3% (1/78). These results demonstrated that coinfection of MiCV with other agents occurs in minks, foxes and raccoon dogs; nevertheless, singular infection is prominent among the animals investigated in the present study. Therefore, whether coinfection of MiCV occurs with pathogens other than E. coli and MEV needs further investigation.
In conclusion, we have revealed, for the first time, that MiCV can also infect foxes and raccoon dogs. The MiCV infection rates in foxes, minks and raccoon dogs were very high on some fur farms in China. The fox was the most sensitive animal to viral infection, followed by the mink and the raccoon dog. Concurrent infections of MiCV and other pathogens may contribute to the disease in minks, foxes and raccoon dogs.
-
This work was financially supported by the National Key Research and Development Program of China (No. 2017YFD0500103), the National Natural Science Foundation of China (No. 31772747), the Science and Technology Development Project of Jilin Province (No. 20170623043TC), the Program for JLU Science and Technology Innovative Research Team (JLUSTIRT, No. 2017TD-28), and the Fundamental Research Funds for the Central Universities.
HTML
Acknowledgements
-
The authors declare that they do not have any conflict of interest.
-
All animal experiments were approved by the Animal Care and Ethics Committee of Jilin University (Changchun, China).
Conflict of interest
Animal and Human Rights Statement
-

Table Supplementary Table S1. Geographic locations of the minks, foxes and raccoon dogs collected in this study
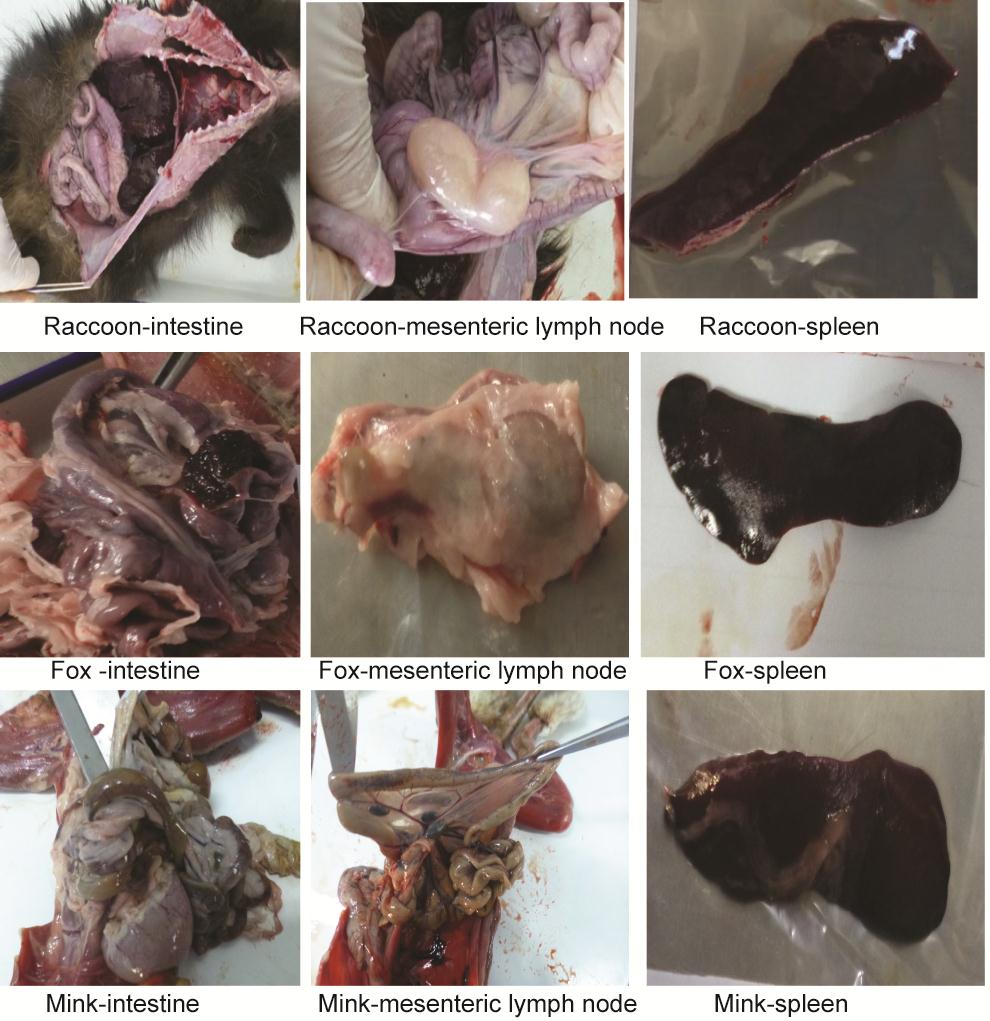
Figure Supplementary Figure S1. The main pathological changes caused by circovirus infection in minks, foxes and raccoon dogs. The main pathologies of the sick animals were enlarged mesenteric lymph nodes and visceral hemorrhages (including those of the gastrointestinal mucosa, liver, spleen and kidney), followed by degenerative necrosis

















 DownLoad:
DownLoad: