HTML
-
Honokiol is a component of the genus Magnolia, which is extensively used in Traditional Chinese Medicine in the form of herbal tea. In recent years, the multifunctional activity of honokiol has attracted considerable attention in preclinical research, especially in cancer therapy. The earlier study identifies honokiol as an active anxiolytic agent in the extract of oriental herbal medicine Saiboku-to (Maruyama et al. 1998; Kuribara et al. 1999, 2000). Subsequently it has been demonstrated that honokiol has antiinflammation, antiangiogenesis and antitumor activity against a variety of tumors, including breast cancer, lung cancer, pancreatic cancer, prostate cancer and other cancers (Bai et al. 2003; Liou et al. 2003; Shigemura et al. 2007; Wolf et al. 2007; Fried and Arbiser 2009; Pan et al. 2014; Zhang et al. 2015; Averett et al. 2016; Song et al. 2016). The underlying mechanisms of honokiol anticancer activity mainly involve dysregulation of cancer-associated signaling pathways and induction of cell death (Xu et al. 2011; Tian et al. 2012). The broad-ranging antitumor activity of honokiol in vitro and in preclinical models would facilitate the development of effective honokiol analogs for targeted cancer therapy.
Previous studies have also demonstrated the impact of honokiol on microbial infection. Honokiol not only inhibits various human pathogenic fungi infection (Bang et al. 2000; Jin et al. 2010; Jacobo-Salcedo Mdel et al. 2011) and functions as an antibacterial agent (Park et al. 2004; Li et al. 2016; Sakaue et al. 2016), also blocks hepatitis C virus (HCV) infection by targeting cell entry and viral replication (Lan et al. 2012) and dengue virus type 2 infection (DENV-2) by inhibiting viral replication, gene expression and endocytotic process of DENV-2 (Fang et al. 2015). However, honokiol shows weak activity against HIV-1 in human lymphocytes (Amblard et al. 2006). Here we assessed the effect of honokiol on herpesvirus herpes simplex virus-1 (HSV-1) infection. We showed that honokiol not only inhibited HSV-1 viral replication and gene expression, also intensified inhibitory effect on HSV-1 infection when being combined with clinical anti-herpesvirus drug acyclovir. Furthermore, honokiol also inhibited the infection of murine gammaherpesvirus 68 (MHV68) and Kaposi sarcoma-associated herpesvirus (KSHV). These results implicate that honokiol not only could be a new candidate for the development of the novel HSV-1 treatment, could also be used for the treatment of other herpesviruses.
-
Vero cells (African green monkey kidney epithelial cells) were cultured in complete Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco). KSHV-positive iSLK endothelial cells were kindly provided by Dr. Ke Lan (Wuhan University) and cultured in DMEM containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) in the presence of 1 μg/mL puromycin (Sigma), 50 μg/mL G418 (Sigma) and 10 μg/ mL hygromycin (Invivogen). The embryonic fibroblasts cells (MEFs) were prepared from embryos E13.5 of C57BL/6 mice. The isolated embryos were minced and typsinized for 20 min, followed by culturing in DMEM medium containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco). The MEFs were passaged two or three times to obtain a morphologically homogenous culture for further usage. HSV-1-GFP virus was generated by introducing a green fluorescent protein (GFP) gene into the intergenic region between UL37 and UL38 of the HSV-1 genome (Li et al. 2011), and was kindly provided by Dr. Chunfu Zheng (Fujian Medical University). HSV-1-GFP viruses were propagated in Vero cells and titered by endpoint dilution assay. MHV68 viruses were propagated in NIH3T12 cells. KSHV RGB-BAC16 viruses were kindly provided by Dr. Ke Lan (Wuhan University). Honokiol (H1309, > 95.0%) was from Tokyo Chemical Industry. Acyclovir (114798-25MGCN) was from Merck Millipore. MHV68 lytic antigen was previously described (Tan et al. 2016).
-
HSV-1 virus titer was measured by 50% tissue culture infective dose (TCID50) method as described elsewhere (Hafidh et al. 2015). Briefly, Vero cells (1.5 × 104 cells/well) were cultured in 96-well plates. After 24 h incubation, the medium was removed, and the monolayer cells were incubated for one hour at 37 ℃ with serial 10-fold diluted HSV-1-GFP virus suspensions in DMEM culture medium. After adsorption, the inoculum was removed, and complete DMEM culture medium (100 μL/well) was added. All plates were incubated at 37 ℃ in a humidified 5% CO2 incubator and were observed daily for cytopathic effect. Estimation of the endpoints was made after 48 h. Titers were calculated as TCID50/mL using the Spearman-Karber method (Darling et al. 1998). The titration was repeated for three times with four replicates for each virus dilution at each time.
Plaque assay was also performed to measure HSV-1 virus titer. Briefly, 200 μL of virus supernatant in 10-fold serial dilution was added to a monolayer of Vero cells in a 6-well plate. After 1 h of incubation at 37 ℃ in 5% CO2, the viral inoculum was aspirated, and 5 mL of 1.5% methylcellulose in DMEM supplemented with 10% FCS was added to each well. After 7 days of incubation, the cells were fixed with methanol/acetate acid (3:1) and stained with 2% crystal violet. The plaques were counted, and the virus titer in plaque-forming units per mL (PFU/ mL) was calculated. Each sample has triplicate.
Plaque reduction assay was performed as previously described (Leary et al. 2002; Tan et al. 2004). Briefly, plaque reduction assays were conducted on the monolayer of Vero cells overlaid with 1.5% methylcellulose (Sigma) for 7 days. The cells were fixed with methanol/acetate acid (3:1) and stained with 0.5% crystal violet prepared in 70% methanol. The viral plaques were counted, a polynomial of order three was used to approximate the data and extrapolate the concentration of drug that inhibits 50% of plaques in each well (IC50). Each sample has triplicate.
-
Genomic DNA was isolated from HSV-1-GFP infected Vero cells. Real-time quantitative PCR (qPCR) was performed to measure HSV-1 viral DNA as previously described (Tan et al. 2016). Total RNA isolation, cDNA synthesis and quantitative RT-PCR (qRT-PCR) to measure HSV-1 viral gene expression were performed as previously described (Tan et al. 2016). The primers for HSV-1 ICP8, ICP27, VP16, and 18S rRNA were described elsewhere (Sato et al. 2016). Primers corresponding to ICP27 gene was used to measure HSV-1 viral genome, the relative genome copy numbers were calculated based on the normalization with the housekeeping gene GAPDH. The primers for GAPDH were: 5'-AACGACCCCTTCATTGACCT-3' and 5'-ATGTTAGTGGGGTCTCGCTC-3'. The relative amount of ICP27, ICP8, and VP16 mRNA expression were normalized to the amount of 18S rRNA expression.
-
Western blot analysis was carried out by standard procedure. The primary antibodies against HSV-1 gD (DL6) sc-21719 and VP16 (vA-19) sc-17547 were purchased from Santa Cruz Biotechnology. HSV-1 ICP27 (ab31631) antibody was from Abcam.
Vero cells were treated with DMSO or honokiol (5 μg/mL and 10 μg/mL) in the presence or absence of acyclovir and subjected to HSV-1-GFP virus infection. HSV-1 infected Vero cells with GFP fluorescence were imaged with a fluorescence microscope (Olympus).
-
Vero cells (1.5 × 104 cells/well) were plated in 96-well plate for 24 h and treated with DMSO or 5 μg/mL, 10 μg/ mL, 15 μg/mL, 25 μg/mL, 50 μg/mL honokiol. Each drug concentration had three replicates. After 48 h incubation, MTT assay was performed to analyze the cell viability using Cell Counting Kit-8 (C0037, Beyotime Biotechnology) according to the manufacturer's instruction.
-
Statistical analysis was performed in Prism (Graph Pad Software). Data are reported as mean ± SD. Differences between groups of research subjects were analyzed for statistical significance with two-tailed Student's t-tests. A P value of < 0.05 was considered significant.
Cell Culture, Viruses, and Reagents
HSV-1 Titration by Endpoint Dilution Assay and Plaque Assay, Plaque Reduction Assay
Real-Time Quantitative PCR and RT-PCR
Western Blot Analyses, Antibodies and Fluorescence Imaging
Cytotoxic Assay
Statistical Analyses
-
Honokiol structurally belongs to a class of biphenols and is hydrophobic (Fig. 1A). We first tested the cytotoxicity of honokiol, treated Vero cells with various concentrations of honokiol for 48 h and assessed cell viability by MTT assay. Treatment with 25 μg/mL honokiol elicited a significant percentage of cell death, about 85% as compared to about 25% cell death in 15 μg/mL honokiol-treated cells (Fig. 1B). The doses of 5 μg/mL and 10 μg/mL honokiol were chosen to use in subsequent experiments since both doses induced low cytotoxicity (Fig. 1B). To determine whether honokiol has any effect on HSV-1 infection, Vero cells were infected with HSV-1-GFP at a multiplicity of infection (MOI) of 0.1 in the presence of honokiol or DMSO control. At 48 h post-infection (hpi), the cytopathic effect was clearly observed in DMSO-treated control cells, but not in Vero cells treated with 10 μg/mL honokiol (Fig. 1C). Next, we tested whether honokiol inhibited HSV-1 infection in a dose-dependent manner. Vero cells were infected with HSV-1-GFP at a high MOI of 1 in the presence of increasing concentration of honokiol. The supernatant was collected at 24 hpi and suggested to plaque assays. We did not observe any cytotoxic effect at 24 hpi, the virus yield of HSV-1 dramatically decreased with increasing concentration of honokiol treatment, indicating that inhibition of HSV-1 virus yield by honokiol was dose-dependent (Fig. 1D). The IC50 was determined to be 10.51 μg/mL for honokiol (Fig. 1E). Taken together, our data showed that honokiol inhibited HSV-1 infection.
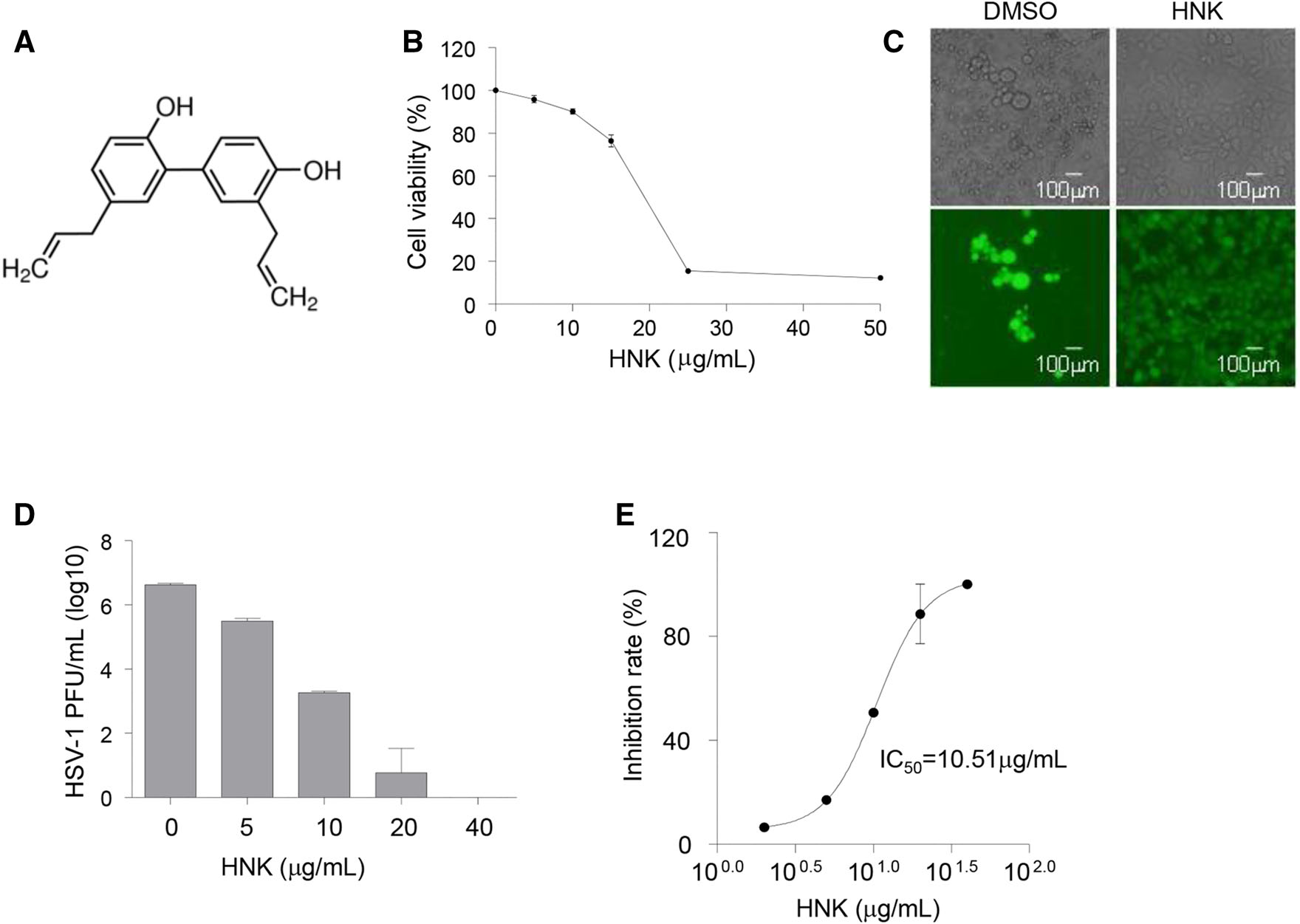
Figure 1. Honokiol inhibits HSV-1 infection. A Structure of honokiol. B Cytotoxicity of honokiol in Vero cells. Vero cells were treated with indicated concentration of honokiol (HNK) for 48 h and subjected to MTT cell viability assay. C Immunofluorescent imaging of HSV-1-GFP infected Vero cells. Vero cells were infected with HSV-1-GFP (MOI = 0.1) for 48 h in the presence or absence of 10 μg/mL honokiol, followed by immunofluorescence imaging. D Virus titer of supernatant from HSV-1 infected Vero cells treated with honokiol. Vero cells were infected with HSV-1-GFP (MOI = 1) in the presence of a serial concentration of honokiol. Supernatant was harvested at 24 hpi and subjected to plaque assay for virus titering. Each sample has triplicate. E Dose-dependent curve for honokiol as determined by plaque reduction assay. IC50 (50% inhibitory concentration) value was calculated by using the fitted functions describing the curve.
-
To determine the underlying mechanism of honokiol inhibition on HSV-1 infection, we tested whether honokiol could block HSV-1 viral DNA replication and gene expression. Vero cells were infected with HSV-1-GFP at an MOI of 0.1 for 8, 16 and 24 h. qPCR analysis was performed to assess HSV-1 viral DNA replication with specific primers corresponding to HSV-1 immediatelyearly gene ICP27 coding region. As compared to DMSO treatment, honokiol treatment significantly decreased HSV-1 viral DNA copy at 8, 16 and 24 hpi (Fig. 2A), suggesting that honokiol inhibited HSV-1 viral DNA replication. qRT-PCR analyses further revealed that gene expression of HSV-1 ICP27, early gene ICP8, and late gene VP16 was markedly increased at both 16 hpi and 24 hpi as compared to early time point 8 hpi in DMSO-treated control cells, however, such an increase was significantly blocked in honokiol-treated cells (Fig. 2B). Next, we tested HSV-1 protein expression by immunoblot analyses with specific antibodies against ICP27 and VP16 at 12 and 24 hpi. Consistent with reduced ICP27 and VP16 mRNA level, both ICP27 and VP16 protein expression were blocked by honokiol treatment (Fig. 2C). Altogether, these data demonstrated that honokiol not only inhibits HSV-1 viral DNA replication, also blocks HSV-1 viral gene expression at both RNA and protein levels.
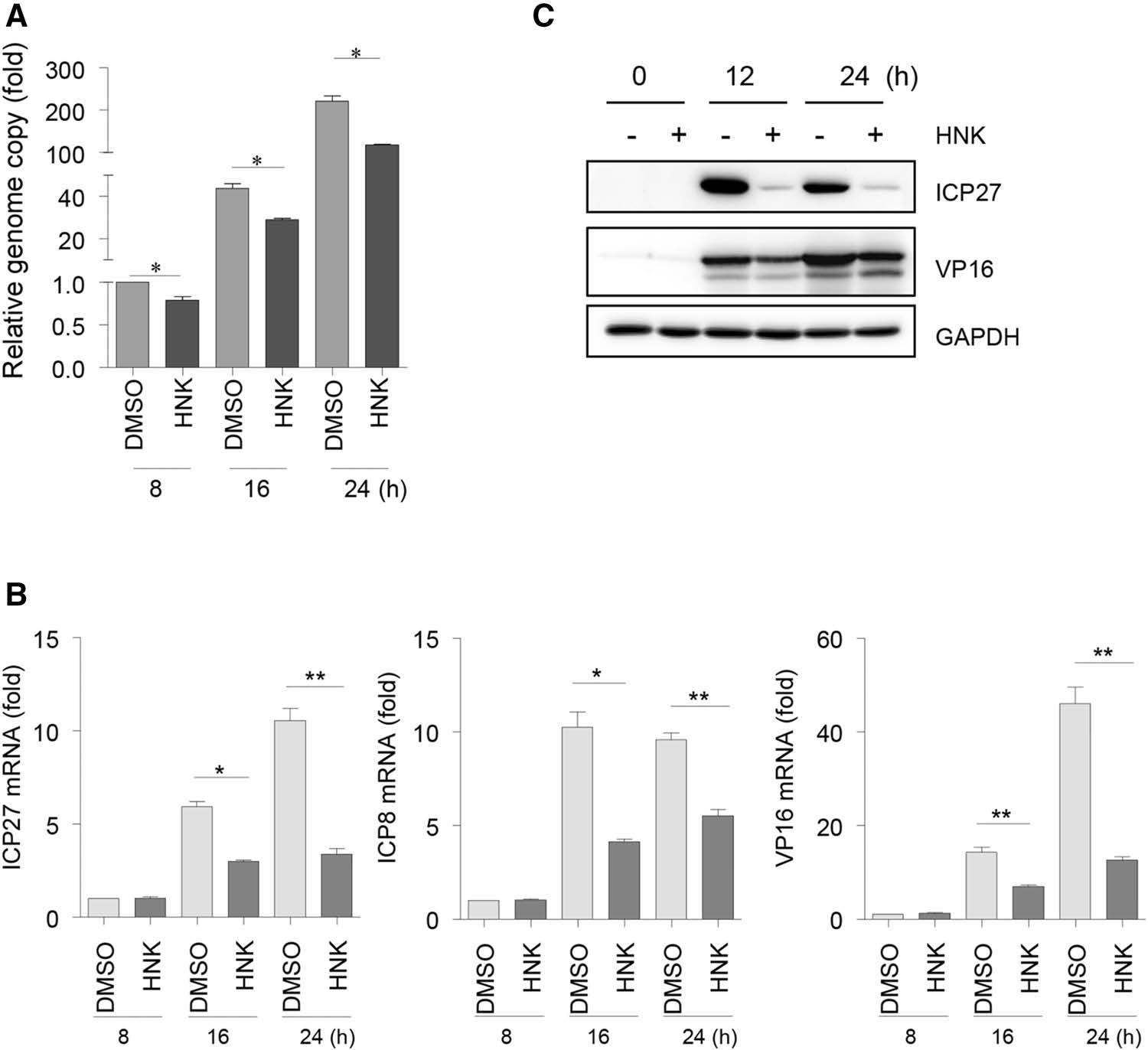
Figure 2. Honokiol inhibits HSV-1 viral DNA replication and gene expression. A qPCR analysis of the HSV-1 viral genome. Vero cells were infected with HSV-1-GFP (MOI = 0.1) for the indicated time in the presence of DMSO or 10 μg/mL honokiol (HNK). Genomic DNA was isolated and subjected to qPCR analysis with primers specific to HSV-1 ICP27 coding region. The relative copy of the viral genome was normalized to GAPDH in each sample. Each data point represents the average (± SD) from triplicate experiments. A P value of < 0.05 (*) was considered significant. B qRT-PCR analysis of HSV-1 viral gene expression. Total RNA was isolated from the same samples as A and subjected to qRT-PCR analysis with the primers specific to HSV-1 ICP27, ICP8, and VP16. Relative RNA amount was normalized to 18S rRNA in each sample. Each data point represents the average (± SD) from triplicate experiments. A P value of < 0.05 (*) or < 0.001 (**) was considered significant. C Immunoblot analyses of HSV-1 gene expression. Vero cells were infected with HSV-1-GFP (MOI = 0.1) in the presence or absence of HNK (10 μg/mL) treatment and harvested at the indicated time point for western blot analysis using a specific antibody against ICP27 or VP16. GAPDH detection was used as a loading control.
-
To determine whether honokiol has any effect on HSV-1 virus entry, Vero cells were pretreated with 10 μg/mL honokiol for 1 h, subsequently infected with HSV-1-GFP at an MOI of 1 and continued to culture in the presence of honokiol. The cells without pretreatment were used as a control, parallelly infected with HSV-1-GFP, and cultured with 10 μg/mL honokiol. There was no significant difference for HSV-1 genome number between the cells with and without honokiol pretreatment at 0, 2, and 4 hpi (Fig. 3A). Next, we collected the infected cells to examine ICP27 mRNA expression at 0, 2, 4, and 6 hpi. Similarly, we did not observe the difference for ICP27 mRNA expression between the cells with and without honokiol pretreatment (Fig. 3B). These data suggest that honokiol has no effect on HSV-1 virus entry.

Figure 3. Honokiol has no effect on HSV-1 virus entry. Vero cells pretreated with (pre) or without 10 μg/mL honokiol were infected with HSV-1-GFP (MOI = 1) for indicated time points. A HSV-1 viral genome determined by qPCR with primers specific to HSV-1 ICP27 coding region. The relative copy of HSV-1 viral genome was normalized to GAPDH in each sample. Each data point represents the average (± SD) from triplicate experiments. B ICP27 mRNA expression determined by qRT-PCR. Relative RNA amount was normalized to 18S rRNA in each sample. Each data point represents the average (± SD) from triplicate experiments. NS represents no significant difference.
-
To further verify whether honokiol impairs HSV-1 virus production and reduce virus titer, Vero cells were infected with HSV-1-GFP at an MOI of 0.005 in the presence or absence of 10 μg/mL honokiol. Virus supernatants were harvested at different time points and used for measuring virus titer by the TCID50 assay. The endpoint virus titers were calculated using Spearman-Karber method. In accordance with aforementioned dose-dependent inhibition of HSV-1 virus yield by honokiol, honokiol treatment reduced virus titers by 102.3–104 folds between 12 hpi and 48 hpi (Fig. 4), further indicating that honokiol has an inhibitory effect on HSV-1 virus production.
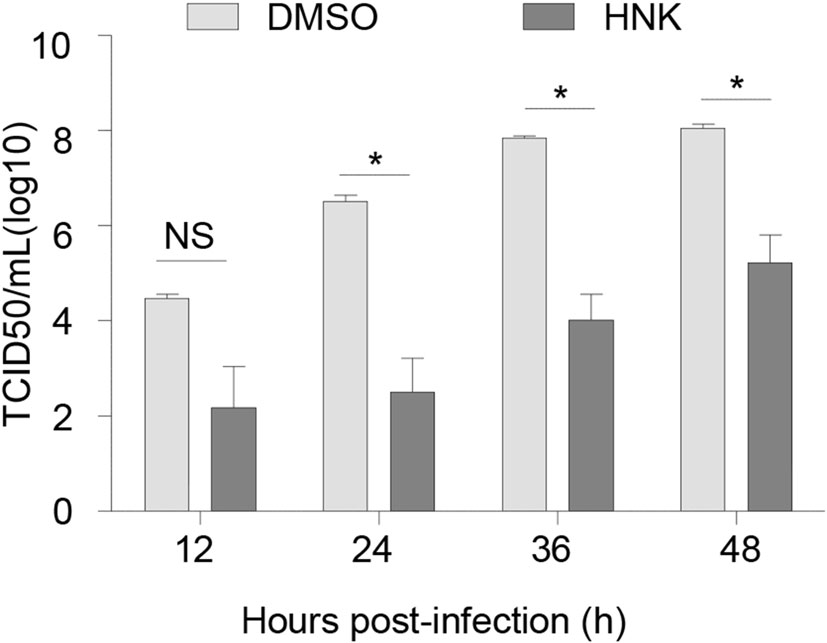
Figure 4. Honokiol blocked HSV-1 virus production. Vero cells were infected with HSV-1-GFP at an MOI of 0.005 in the presence or absence of HNK (10 μg/mL) treatment. The supernatant was harvested at indicated time points and subjected to TCID50 assay for measuring virus titer. Each data point represents the average (± SD) from triplicate experiments. A P value of < 0.05 (*) was considered significant. NS represents no significant difference.
-
Acyclovir is a nucleic acid analog and primarily used to treat HSV and varicella-zoster virus (VZV) infections (Elion 1993; Wutzler 1997). To reveal how combination treatment of honokiol and clinical drug acyclovir influences HSV-1 infection, Vero cells were infected with HSV-1-GFP at an MOI of 0.1 in the presence or absence of a serial of combination treatment of acyclovir and honokiol. Acyclovir or honokiol treatment alone displayed dosedependent antiviral activity against HSV-1, reducing GFP-positive HSV-1 infected cells in a dose-dependent manner (Fig. 5A). Importantly, combination treatment of honokiol and acyclovir not only showed a more reduction of GFP-positive HSV-1 infected cells at 24 hpi as compared to honokiol or acyclovir alone (Fig. 5A), also intensified block of HSV-1 ICP27, VP16 and gD protein expression as detected by immunoblot analyses (Fig. 5B). Combination of 1 μmol/L acyclovir and 5 μg/mL or 10 μg/mL honokiol elicited more effective inhibition in HSV-1-GFP infection and protein expression of ICP27, VP16 and gD than 5 μmol/L acyclovir alone (Fig. 5A, 5B). However, as compared to honokiol or acyclovir alone, the combination of honokiol and acyclovir did not dramatically induce additive or synergistic block for mRNA expression of HSV-1 viral genes and HSV-1 virus titer (Fig. 5C, 5D). Altogether, these results illustrate that honokiol augmented antiviral activity with acyclovir against HSV-1 infection, but the effect of the combination treatment was not simply additive or synergistic, which might result from distinct underlying mechanisms of honokiol and acyclovir antiviral activities. Nevertheless, our data implicate that acyclovir dose could be decreased if using combination treatment with honokiol against HSV-1 infection. Thus the side effect of acyclovir could also be correspondingly reduced. Honokiol could be a potential candidate for the treatment of HSV-1 infection, either acting alone or in combination with other therapeutics like acyclovir.
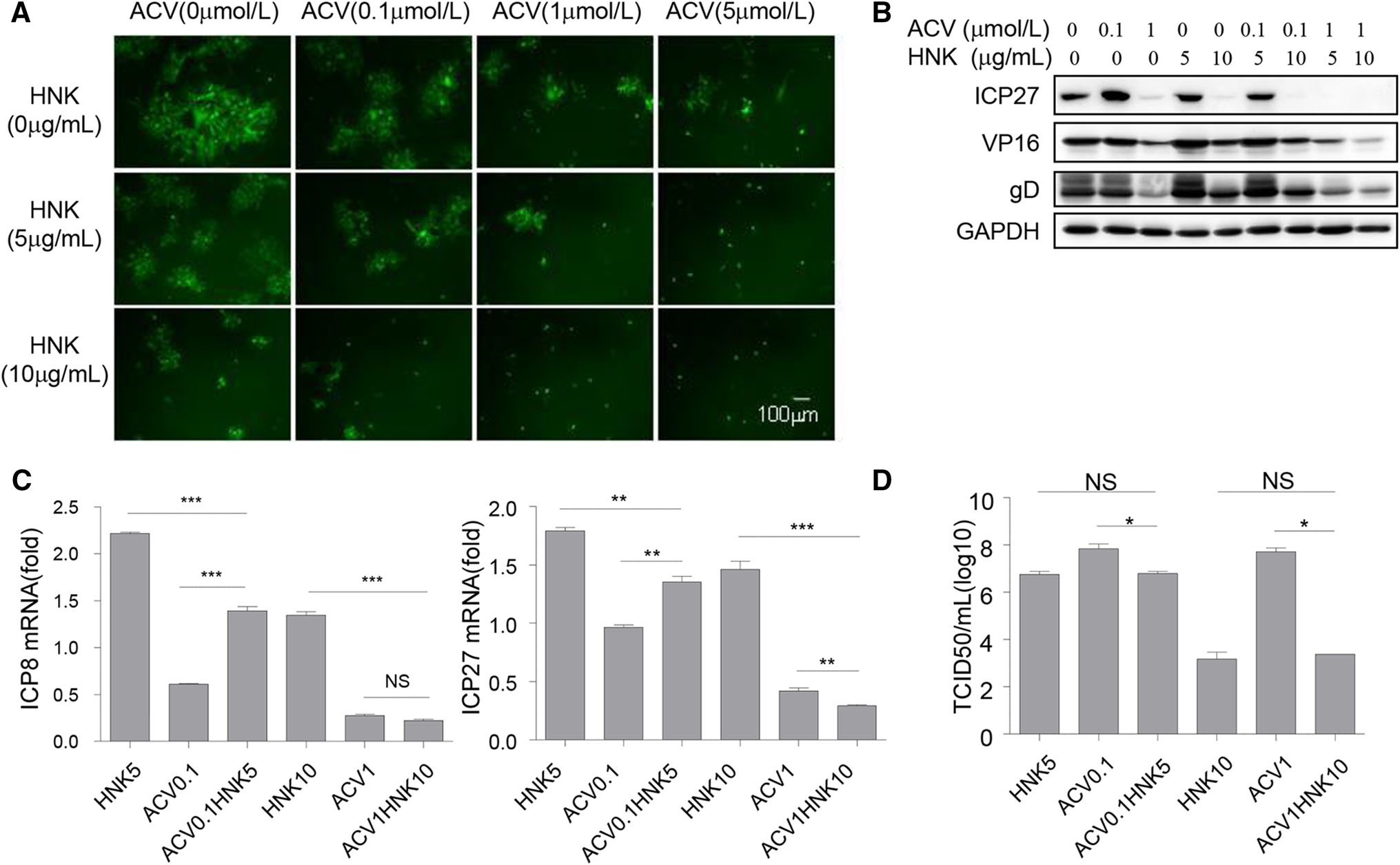
Figure 5. Honokiol enhanced inhibition of HSV-1 infection with acyclovir. Vero cells were infected with HSV-1-GFP (MOI = 0.1) for 24 h following combination treatment of a serial concentration of honokiol and acyclovir. A Immunofluorescent imaging of HSV-1-GFP infected cells. B Western blot detection of HSV-1 gene expression with the specific antibodies against ICP27, VP16, gD and loading control GAPDH. C qRT-PCR analyses of ICP8 and ICP27 mRNA expression. Relative RNA amount was normalized to 18S rRNA in each sample. Each data point represents the average (± SD) from triplicate experiments. A P value of < 0.05 (*), < 0.001 (**) or < 0.0001 (***) was considered significant. NS represents no significant difference. D The supernatant was harvested and subjected to TCID50 assay for measuring virus titer. Each data point represents the average (± SD) from triplicate experiments. A P value of < 0.05 (*) was considered significant. NS represents no significant difference.
-
To assess whether honokiol inhibits other virus infection, primary murine embryonic fibroblasts were infected with murine gammaherpesvirus MHV68 at a low MOI or high MOI in the presence or absence of 10 μg/mL honokiol. The infected cells were harvested at different time points, followed by immunoblot analyses with the MHV68 lytic antigen serum. Honokiol dramatically blocked the expression of MHV68 lytic antigens regardless of infection with the low or high MOI (Fig. 6A). We also examined the effect of honokiol on human gammaherpesvirus KSHV. SLK cells were infected with KSHV RGB-BAC16 viruses whose infection shows RFP red fluorescence in the infected cells. Similarly, honokiol significantly inhibited KSHV infection (Fig. 6B), suggesting that honokiol might have broad-ranging antiviral activity against herpesviruses.
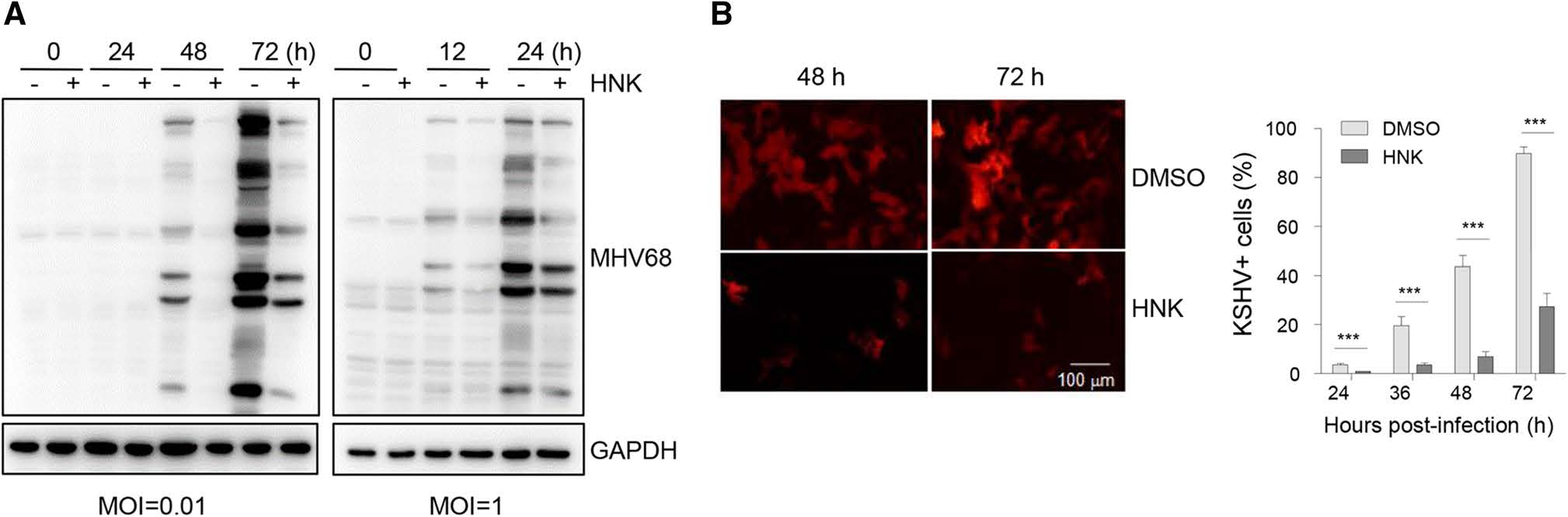
Figure 6. Honokiol inhibits MHV68 and KSHV infection. A MEF cells were infected with MHV68 viruses at an MOI of 0.01 or 1 in the presence or absence of honokiol (HNK) (10 μg/mL) treatment. The infected cells were harvested at the indicated time points and subjected to immunoblot analyses with the MHV68 lytic antigen serum. GAPDH was used as a loading control. B Inhibition of KSHV infection by HNK. SLK cells were infected with KSHV RGB-BAC16 viruses in the presence or absence of honokiol (HNK) (10 μg/mL) treatment. Left panel was the representative fluorescent images of KSHV-infected cells at 48 h and 72 h. Right panel was the statistic data for KSHV-infected cells. Each data point represents the average (± SD) from triplicate experiments. A P value of < 0.001 (**) or < 0.0001 (***) was considered significant.
Honokiol Inhibits HSV-1 Infection
Honokiol Inhibits HSV-1 DNA Replication and Gene Expression
Honokiol has no Effect on HSV-1 Virus Entry
Honokiol Inhibits HSV-1 Virus Production
Honokiol Intensifies Antiviral Activity with Acyclovir against HSV-1 Infection
Honokiol Inhibits the Infection of Other Viruses
-
Honokiol anticancer activity has been extensively investigated, but the study of honokiol antiviral activity remains limited. Only two reports have shown that DENV-2 and HCV infection are inhibited by honokiol with different underlying mechanisms (Lan et al. 2012; Fang et al. 2015). In the current study, we show that honokiol inhibits HSV-1 infection through blocking HSV-1 viral DNA replication and gene expression, the combination of honokiol with clinical antiherpesvirus drug acyclovir enhanced inhibition of HSV-1 infection.
Honokiol induces cytotoxicity and cell death at a high concentration of 40 μg/mL in Vero cells, which is consistent with honokiol anticancer activity reported in other cancer cells and in agreement with honokiol as a potential broad-ranging anticancer therapeutic drug (Fried and Arbiser 2009; Kumar et al. 2013). However, at low concentration with no sign of notable cytotoxicity, honokiol exerts inhibitory effects on HSV-1 infection in Vero cells, implicating that honokiol could be a potential antiherpesvirus drug candidate. In line with previous reports that honokiol blocks DENV-2 and HCV infection (Lan et al. 2012; Fang et al. 2015), but not HIV-1 infection (Amblard et al. 2006), honokiol antiviral activity appears to be virusspecific instead of wide-ranging. Given that honokiol similarly inhibits the infection of MHV68 and KSHV, at least, honokiol might have a broad antiviral effect on herpesvirus infection. It's worth noting that the effective concentration of honokiol against HSV-1 infection is a bit narrow. It would be helpful to develop effective honokiol analogs for better targeted anti-HSV-1 therapy.
Acyclovir is the first effective compound discovered and used in HSV and VZV treatment worldwide clinically (Field and Vere Hodge 2013). Acyclovir itself is inactive, needs both viral thymidine kinase and cellular enzyme to become active, inhibiting viral DNA synthesis (Elion et al. 1977). However, the non-functional viral thymidine kinases resulted from mutation renders some HSV and VZV strains resistant to acyclovir treatment, becoming an emerging problem especially in immunocompromised patients (Balfour et al. 1994; Reusser 1996; Andrei et al. 2012). Honokiol is a lignan extracted from some species of Magnolia and has a fairly limited side effect (Woodbury et al. 2013). The inhibitory effect of honokiol on HSV-1 infection was at the level of viral DNA replication and viral gene expression, not at the level of virus entry. Considering that the transcription of HSV-1 genes is controlled by RNA polymerase Ⅱ of the infected host, it's highly likely that honokiol exerts its effect on RNA polymerase Ⅱ of HSV-1-infected cells, resulting in the inhibition of HSV-1 gene transcription and subsequent DNA replication. Although the mechanisms by which honokiol inhibits HSV-1 viral DNA replication and gene expression need further investigation, nevertheless, the inhibitory effect of honokiol on HSV-1 infection provides a potential drug candidate, which most likely functions independent of viral gene activity and thereby would escape drug-resistant problem like acyclovir. The strengthened inhibitory effect of honokiol and acyclovir also provides a choice of the combination treatment of honokiol and acyclovir again HSV-1 infection. The underlying mechanism of the combination effect was not simply additive or synergistic, which needs to be further illustrated. At least, the combination treatment against HSV-1 infection with honokiol could decrease acyclovir dose and might correspondingly reduce the side effects of acyclovir. However, more preclinical research needs to be carried out before the consideration for clinical treatment of honokiol in HSV-1 infection. We currently demonstrate the antiviral activity of honokiol against HSV-1 infection, providing a promising therapeutic candidate for HSV-1 infection. In the future study, we attempt to evaluate the efficacy of honokiol in a mouse model.
-
We thank Dr. Chunfu Zheng (Fujian Medical University) for providing the HSV-1-GFP virus. This work was supported by the grant from National Key Research and Development Program (2016YFA0502100).
-
XL conceived and designed the experiments. SL, LL and LT performed the experiments and analyzed the data. XL wrote and finalized the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
All animal studies were conducted following protocols approved by the Institutional Animal Care and Usage Committee of Institut Pasteur of Shanghai, Chinese Academy of Sciences.














 DownLoad:
DownLoad: