HTML
-
African swine fever (ASF) is a potent infectious disease caused by ASFV, leading to 100% mortality in acute infections. Since the first report of ASF in Kenya in 1921, dozens of countries and regions have erupted already (Luther et al. 2008). China announced the first ASF epidemic in August 2018, and multiple outbreaks had been reported currently (Zhao et al. 2019). ASFV had posed a significant threat to the swine industry worldwide, causing deadly substantial economic losses.
ASFV targets porcine macrophages via clathrin and cholesterol-dependent endocytosis (Galindo et al. 2015) to initiate infection, leading to high fever, severe depression, anorexia, skin redness, congestion, cyanosis, peripheral blood platelets and lymphocytes decreasing in swine (Chen et al. 2016). However, there still remains gaps in understanding and recognition of ASFV due to the complexity of the virus, existence of multiple genotypes, and limited knowledge about protective immunity, seriously hindering the development of effective vaccines against ASFV (Rock 2017).
So far, no commercially effective vaccine or drug is available for preventing, controlling and treating ASFV. General antiviral vaccines can be divided into inactivated vaccines, live attenuated vaccines (Ohmit et al. 2006) and subunit vaccines (Zhang et al. 2013). In addition to utilize the virus as a vaccine traditionally, ASFV subunit vaccine adopting specific antigens as the core has been extensively studied. A recent study had proved that a pool of vaccinia virus-vectored ASFV antigens delivered by a subunit vaccine was not only able to significantly achieve partial protection but also reduce viral genome loads in blood and lymphatic tissues (Jankovich et al. 2018). Besides, other promising outcomes such as delayed onset of viremia, delayed mortality had been confirmed using either one or a combination of a few antigens (Argilaguet et al. 2013). Thus, the development of subunit vaccines is possible, but it requires incorporation of multiple protective antigens.
After carrying out a large screening of ASFV-encoded antigens, we selected and synthesized two important viral genes p30, p54 as candidate genes for yeast vaccine eventually, in order to perform the oral-immunization with a cocktail of mix-antigens in swine. In detailed, specific ASFV antigens P30 and P54, which have been tested as vaccine candidates, are capable of neutralizing ASFV after attacking susceptible cells and also inhibiting the process of virus attachment, replication and internalization during ASFV infection (Stedman 2017). A delay in onset of clinical symptoms and reduced viremia were observed in pigs immunized with both P54 and P30 together, and a half of pigs survived after virulent ASFV challenge. Meanwhile, immunization with P54 or P30 antigen alone was not sufficient to protect pigs against virulent ASFV challenge (Barderas et al. 2001; Parisotto et al. 1998). Similarly, after immunization with baculovirus-expressed fusion protein comprising three in tandem ASFV antigens (P54, P30 and the extracellular domain of the viral hemagglutinin), four out of six pigs of the immunized group also remained viremia-free after ASFV infection (Argilaguet et al. 2013). Taken together, these indicated that partial to complete protection in swine had been proved following immunization with a cocktail of recombinant P30 and P54 protein.
On the other hand, cell-mediated response is also important in protection against ASFV in addition to neutralizing antibodies (Ruiz-Gonzalvo et al. 1996). Pigs immunized with an attenuated ASFV strain and non-depleted of CD8+ T cells were protected from homologous virulent challenge, whereas pigs depleted of CD8+ lymphocytes were no longer completely protected from virulent ASFV challenge, suggesting the importance of the cell-mediated immune response against ASFV (Oura et al. 2005). Thus, vaccines capable of eliciting both humoral and cell-mediated immune responses are required urgently for conferring protection against ASFV. Fc fragments of immunoglobulin have been verified to elicit a broad range of biological functions such as activating of complement, pluripotent cells, passaging through the placenta, participating in antigen presentation and processing, and binding to bacterial proteins (Frommel and Hong 1970). The Fc receptor, a member of the immunoreceptor family, presented on the surface of various immune effector cells, would bind to Fc fragments of immunoglobulin specifically. Antibodies binding to a specific antigen interacts with the Fc receptors on the cell surface through its Fc segment, thereby stimulating the body to produce various immune responses such as phagocytosis, endocytosis/exocytosis, oxidative burst, antibody-dependent cytotoxicity (Mendez-Fernandez et al. 2010). It is known that the Fc receptors allows the fetus or newborn to obtain maternal IgG through the placenta or intestinal route (Vaughn and Bjorkman 1997). Furthermore, the receptor also transports IgG antibodies through the mucosal surface in adults at the age of 9–12 years old and develops resistance to intestinal pathogens (Yoshida et al. 2007). Since neonatal Fc receptor-mediated IgG transport across the mucosal epithelium potentially provide the mucosal protection (Guo et al. 2016), the fusion of antigens with Fcγ or Fcα fragment, a recently-developed novel vaccine strategy, may allow Fc receptors to mediate antigen transmembrane barrier transport, integrating humoral and cellular immunity effectively.
Despite of identification of protective antigens, the other main requirement for development of subunit vaccines is a delivery system capable of eliciting protective immunity. Since the development of the filamentous phage display technology, various surface display systems such as λ phage (Niwa et al. 2000), T4 phage (Gamkrelidze and Dąbrowska 2014), T7 phage (Ludtke et al. 2007), bacteria (Klemm and Schembri 2000), baculovirus (Song et al. 2010), yeast (Kim and Kim 2016) have been developed. As a powerful eukaryotic display system for immobilized heterologous protein, the S. cerevisiae surface display system has overcome the shortcomings of eukaryotic protein activity or inactivation when expressed in prokaryotic cells (Fujita et al. 2002), and the yeast surface display technology has been shown to distinguish well between mutants with weak affinity differences (Sivelle et al. 2018).
Vaccines against mucosal pathogens are supposed to induce mucosal and potential systemic immune responses simultaneously to prevent infectious agents from entering the mucosal surface (Negri et al. 2010). A previous study had revealed the close relationship between mucosal epithelial cells and immune effector cells in the lamina propria, suggesting that immunogens conveyed by oral vaccines through the mucosal surface might be an ideal approach to stimulate protection against ASFV infection (Kiyono and Azegami 2005). S. cerevisiae has been popularly used in recombinant antigen proteins production and serve as the delivery platform recently in the pharmacomedical field for the reason that S. cerevisiae can regulate the balance of intestinal flora to stimulate mucosal immunity and systemic immunity subsequently (Olson et al. 1996). Notably β-glucans are key component of the cell walls of S. cerevisiae, which would be released after biodegradation of yeast particles by phagocytes that migrated to the mesenterial lymph nodes (De Smet et al. 2014). It had been proved that β-glucans serve as potent activators of the innate immune system to against the infection of bacterial, viral, and fungal diseases (Markova et al. 2003; Wang et al. 2008; Luzio and Williams 1978) by enhancing the functional activity of neutrophils, macrophages, dendritic cells, and epithelial cells (Olson et al. 1996). In particular, β-glucans possessed beneficial effects on T- and B cell responses (Leibundgut-Landmann et al. 2007), which would help stimulate cell-mediate immune response after immunization with oral vaccine against ASFV. What's more, it is generally accepted that the S. cerevisiae surface display system is particularly suitable for the selection of antigen-binding Fc fragments since the system allows rapid combinatorial library construction via gap repair-driven homologous recombination and an efficient display of a glycosylated Fc segment which would interact with Fc receptors (Wozniak-Knopp et al. 2017). In summary, along with its adjuvant properties, S. cerevisiae is equipped with a large antigen payload and antigen sparing capacity as an oral vaccine delivery platform. In this study, we developed a new vaccine strategy against ASFV based on S. cerevisiae surface display system, providing potent vaccine options for swine health worldwide.
-
Peripheral blood mononuclear cell (PBMC) was isolated from pigs of Tianjin Ninghe original swine farm according to a previously described protocol (Chen et al. 2018). PAM cell lines CRL2843-CD163 (3D4/21) were grown in RPMI-1640 medium (Gibco, ThermoFisher Scientific, USA) supplemented with 10% (V/V) fetal bovine serum (FBS, Biological Industries) and antibiotic–antimycotic solution (Gibco, ThermoFisher Scientific, USA). IPEC-J2 cells were cultured in Dulbecco's modified essential medium (DMEM) containing 10% fetal bovine serum. 3D4/21 and IPEC-J2 cells were both passaged and preserved by our laboratory according the protocol.
-
Total RNAs were extracted from PBMC cells using TRIzol reagent (TaKaRa, Beijing). First-strand cDNA synthesis was carried out using reverse transcriptase (TaKaRa, Beijing). IgG1 and IgA1 genes were synthesized by PCR and the specific primers (Supplementary Table S1) based on the predicted IgG1 and IgA1 sequences revealed at NCBI (GenBank accession: No. AK405786.1 and No. AB194101.1), and the amplified fragments recycled by TIAN gel midi purification kit (TIANGEN, Beijing) after electrophoresis in 1% (w/v) agarose/TAE gel were cloned into pGEMⓇ-T Easy Vector (Transgen, Beijing) through ligation reaction. The heavy chains of IgG1 and IgA1, Fcγ and Fcα were amplified by PCR to fuse with ASFV genes by overlapping PCR.
-
Codon optimization, ASFV gene synthesis were all outsourced (GENEWIZ, Beijing), as well as cloning into pMD19T and gene sequence validation. The resultant amino acid sequences of ASFV antigens p30/p54 after gene codon optimization were beneficial for recombinant proteins expression in yeast. The genes containing the codonoptimized cDNA sequence of full-length p30/p54 were ligated with Fcγ or Fcα to obtain p30-Fcγ and p54-Fcα tandem genes by overlapping PCR with specific primer pairs harboring homologous sequences with the vector. Then p30-Fcγ and p54-Fcα tandem genes were sub-cloned into the yeast expression vector pGPD-ADH1-POT respectively with a His tag at the C terminal by using a one-step cloning kit (Vazyme, Nanjing), which would grant the expression cassettes of all antigens on the surface of yeast but also allow for tracking expression, affinity purification of recombinant proteins by the tag-specific antibody. The two resulting plasmids were named pGPD-p30-Fcγ-ADH1-POT, pGPD-p54-Fcα-ADH1-POT, and used to transform the host S. cerevisiae strain, ST1814G (Fig. 1). Fusion gene fragments p30-Fcγ and p54-Fcα contained in two transcription units were introduced into the genome of two S. cerevisiae strains ST1814G respectively by LiAc transformation. Meanwhile, these two fusion gene fragments in proper order were tandem transformed into one S. cerevisiae strain in order to express P30 and P54 proteins simultaneously. Then, p30-Fcγ and p54-Fcα genes with a selective leucine (LEU) marker were integrated into the chromosome IV of yeast replacing the pre-existing selective marker at the HO locus. The recombinant S. cerevisiae strains producing LEU are capable of growing in SD-LEU auxotrophic medium regularly while the host S. cerevisiae strain ST1814G lacking of the ability to produce LEU would reach the end of proliferation.
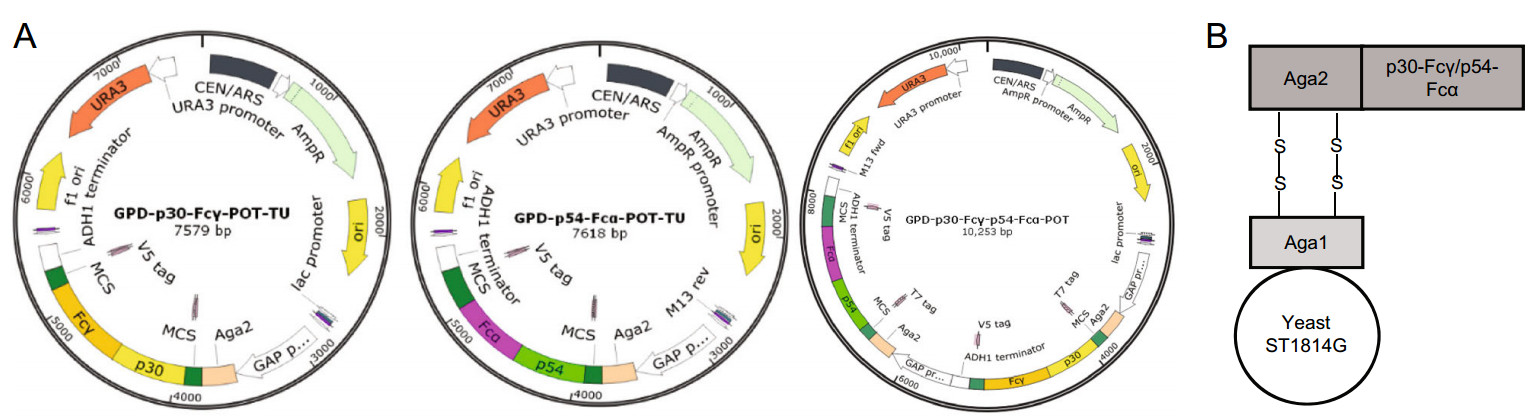
Figure 1. Construction of p30-Fcγ, p54-Fcα, p30-Fcγ-p54-Fcα expression vectors. A Diagram of the chromosomal region, including the p30-Fcγ and p54-Fcα open reading frame. GAP promoters are indicated as white arrow, ADH1 terminators are shown as white rectangle. Aga2 which would combine with Aga1 expressed on the surface of ST1814G is indicated in peach puff. B A schematic diagram showing the recombinant S. cerevisiae strain expressing ASFV proteins.
As for the purification of prokaryotic proteins expression, the ASFV genes p30/p54/k145r were cleaved with BamHI (Takara, Beijing) and visualized using an agarose gel and the target fragments were recovered and recombined into the pET28 vector by using a one-step cloning kit (Vazyme, Nanjing). The resulting prokaryotic expression vectors pet-28a-p30, pet-28a-p54, pet-28a-k145r were constructed using primer pairs pet-28a-p30-F/R, pet-28a-p54-F/R and pet-28a-k145r-F/R. The constructs generated were validated by DNA sequencing (GENEWIZ, Beijing). The recombinant vectors coding for His-P30/P54/K145R was then transformed into Escherichia coli (E. coli) BL21 DE3 competent cells (Transgen, Beijing) respectively for prokaryotic protein expression. All His-ASFV proteins were expressed and purified by Ni–NTA agarose (ThermoFisher Scientific, Beijing) using the same procedures as already described by Crowe (Crowe et al. 1994). Detailed sequences of the primers used in the constructions are listed in Supplementary Table S1.
-
The S. cerevisiae strain ST1814G utilized as the yeast surface display system was routinely cultured in yeast extract peptone dextrose (YEPD/YPD) containing 20 g/L glucose, 20 g/L tryptone, and 10 g/L yeast cells extract (Sangon biotech, Shanghai). Plasmid vectors were transformed into host yeast cells with classical lithium acetate (LiAc) method as previously described by Gietz and Woods (Gietz and Woods 2002). S. cerevisiae transformants were selected on synthetic minimal SD media containing appropriate auxotrophic supplements (Supplementary Figure S1A). The individual transformants picked out were inoculated into 3 mL of YPD medium and grown at 30 ℃ with shaking for 24 h on an orbital incubator. Yeast cells were harvested by centrifugation for genomic DNA extraction subsequently. The genomic DNA was subjected to PCR analysis by p30-Fcγ/p54-Fcα-specific primer pairs to confirm the correct recombination of two transcription units (Supplementary Table S1, Figure S1B). PCR products were run on a 1% (w/v) agarose/TAE gel. Expression of recombinant proteins was induced at 30 ℃ for 24 h. After induction, all recombinant S. cerevisiae strains were preserved using 30% glycerol and stored in a - 80 ℃ freezer prior to use.
-
Yeast cells were harvested by centrifugation and resuspended in PBS. Then yeast cells were frozen and thawed three times using liquid nitrogen to obtain cell lysates and total RNAs of yeast cells were extracted using TRIzol reagent (TaKaRa, Beijing). First-strand cDNA was synthesized from purified RNAs of yeast using a First-Strand Synthesis System (Transgen, Beijing) according to the manufacturer's instructions. The relative gene expression analyzed by qRT-PCR was performed on an ABI 7500 Real-time PCR system (Applied Biosystems, Foster city, CA, USA). The comparative cycle threshold (CT) method was used to calculate the relative gene expression level according to manufacturer's protocol (Applied Biosystem). All data presented was relatively quantitative based on the mRNA level of the endogenous gene ACT1 and analyzed using GraphPad Prism 6.0 software. All of the primers pairs used for qRT-PCR were listed in Supplementary Table S2.
-
To examine the expression of P30-Fcγ and P54-Fcα fusion proteins of three recombinant S. cerevisiae strains, protein extracts were separate by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, ThermoFisher Scientific, USA). After cultivation for 24 h, yeast cells were divided into supernatant and pellet by centrifugation at 3000 × g for 2 min at 4 ℃, and the pellet of recombinant S. cerevisiae cells were collected for protein preparation. Then yeast cell lysates after pretreatment with b-mercaptoethanol (Gibco, ThermoFisher Scientific, USA) were dissociated in buffer (2% SDS, 100 mmol/L dithiothreitol, 125 mmol/L Tris–HCl, pH 6.8), heated at 90 ℃ for 10 min and the samples were stored at - 80 ℃ until electrophoresis with 12% SDS–PAGE. The separated proteins were transferred to PVDF membrane which were activated by methanol. Then proteins-enriched membranes were blocked with PBS (0.1% Tween-20) added 5% (w/v) Bovine Serum Albumin (BSA) for 1 h and incubated with His-specific antibody (Santa Cruz, CA) as the primary antibody (1: 5000 dilution) overnight at 4 ℃, followed by incubation with HRP (Horseradish peroxidase)-conjugated secondary antibody (Invitrogen, USA) for 1 h at room temperature. After each step, yeast cells were washed at least three times with PBS. Bound antibodies were detected using Pierce ECL Western Blotting Substrate (ThermoFisher Scientific, USA) and band densitometry was performed with Image Lab software (BioRad). Data were normalized to control values.
-
Yeast cells were fixed with 4% paraformaldehyde (Takara, Beijing) for 15 min and washed with PBS for 3 times at 21 ± 4 ℃, and then blocked with PBS (0.1% Tween-20) added 5% (w/v) BSA for 30 min at 21 ± 4 ℃ and incubated with primary antibody (anti-His, 1: 5000 dilution) overnight at 4 ℃ in a humid chamber. After the washing step with PBS, the secondary antibody FITC-conjugated anti-mouse IgG (1: 200 dilution) was incubated for 1 h at 21 ± 4 ℃. For immunofluorescence analysis, yeast cells were seeded on glass coverslips, and cover slips were mounted on slides. The localization of P30-Fcγ and P54-Fcα fusion proteins were observed with the confocal microscope (UltraView Vox, PerkinElmer, USA). Images were taken at × 100 magnification and subsequently processed with Volocity Demo software.
-
Characterization of the three recombinant S. cerevisiae strains by flow cytometry has been based on the set of His tag marker as described above. The experiment procedure was similar to the one described in immunofluorescence. Yeast cells were harvested after 24 h and washed three times with PBS. Then yeast cells were fixed with 4% paraformaldehyde for 15 min and then washed with PBS for 10 min at 21 ± 4 ℃. Nonspecific binding was blocked with PBS (0.1% Tween-20) added 5% (w/v) BSA for 30 min and then incubated with primary antibody (antiHis) overnight at 4 ℃ in a humid chamber. Cells were incubated the secondary antibody (anti-mouse IgG, 1: 200 dilution) directly conjugated to fluorescein isothiocyanate (FITC), washed, transferred to PBS buffer. Fluorescenceactivated cell sorting was performed at the Umea Immunology Center on a FACS LSR II (BD Biosciences, USA), and a total of 1.5 × 105 cells were analyzed. Data were analyzed using FlowJo software (Tree Star).
-
Animal experiments were performed in accordance with the relevant guidelines and regulations. Yeast cells were harvested in the exponential growth phase and pigs at the age of 8–9 weeks were divided into 2 groups with 10 individuals in each group. All pigs were provided with standard swine food and water ad libitum. The immunization protocol for groups A was as following: pigs were oral vaccinated with 100 μL of PBS and used as a negative control. Meanwhile, each pig in group B was fed with a cocktail of three recombinant S. cerevisiae strains expressing ASFV antigens about 109 CFU resuspended in 20 mL PBS throughout the whole experiment progress. Three boost immunizations with the same amount of recombinant yeast cells were conducted in 2-week interval and therefore the whole experiment lasted 8 weeks. The different serum samples of control and immunized pigs collected before and after immunization were stored in - 80 ℃ after classification and used to determine the immune response.
-
The levels of antigen-specific IgG and IgA in swine serum collected at 2 weeks post-boost were determined by ELISA method. Briefly, 96-well microplates coated overnight with 2.5 μg/mL (100 μL/well) of recombinant affinity-purified ASFV antigens P30/P54/K145R in bicarbonate coating buffer and then blocked with PBS (0.1% Tween-20) added 5% (w/v) BSA. Then the microplates were incubated (in triplicates) with 2 fold serum dilutions starting from 1:4000 to 1:8 × 106. Pre-immunization serum similarly diluted served as a positive control, whereas normal pig serum (1:200 dilution) was used as a negative control. Then the microplates were incubated with 100 μL peroxidase-conjugated anti-swine IgG/IgA (Cell Signaling Technology, CST, USA) and were developed using Sure Blue Reserve TMB substrate. The substrate was reacted at room temperature in the dark for 10 min, and the reaction was stopped using 1 N hydrochloric acid. The optical density (OD) at 450 nm was then determined using a microplate spectrophotometer. The levels of antigen-specific antibodies were determined by calculating the positive/negative (P/N) value. The significance of the difference in antigen-specific IgG/A titers among the groups was determined by analysis of variance (ANOVA) followed by Tukey's multiple-comparison test and a P value of < 0.05 was considered significant.
-
The experimental data were subjected to one-way analysis of variance (one-way ANOVA) and expressed as mean ± SEM. Pairwise multiple comparison was conducted to determine which group differed by two-way ANOVA followed by Bonferroni post-tests using Prism 6.0 (GraphPad Software Inc.). All results are presented as mean ± standard deviation (SD) from at least three independent experiments and a difference were considered statistically significant if a P value < 0.05.
Cells
The Heavy Chain Gene Cloning of Porcine IgG1 and IgA1
Plasmid Construction
Yeast Strain, Media and Culture Conditions
Quantitative Real-Time PCR (qRT-PCR)
Western Blotting
Immunofluorescence Assay
Flow Cytometry Analysis
Immunization of Swine
Enzyme-Linked Immunosorbent Assay (ELISA)
Data Analysis
-
Codon-optimized synthetic genes encoding ASFV antigens P30 and P54 fused with Fcγ and Fcα respectively in frame were used to generate pGPD-POT constructs and recombinant S. cerevisiae strains. The open reading frame (ORF) of the p30-Fcγ and p54-Fcα fusion genes obtained by overlapping PCR were cloned into the plasmid vector pGPD-POT including a glucose promoter GPD for expression in S. cerevisiae strain ST1814G as previously described in our laboratory (Chen et al. 2018), and a His tag as the filter mark for later detection which has been knocked out in the genome of ST1814G. The schematic diagram of the expression vectors pGPD-p30-Fcγ-ADH1-POT, pGPD-p54-Fcα-ADH1-POT and pGPD- P30-Fcγ-P54-Fcα-ADH1-POT is shown in Fig. 1A.
We successfully screened and constructed three different recombinant yeast strains expressing the selected ASFV antigens with GPD promoter including two single expression recombinant S. cerevisiae strains named ST1814G/P30-Fcγ, ST1814G/P54-Fcα respectively, and one tandem composite recombinant S. cerevisiae strain named ST1814G/P30-Fcγ-P54-Fcα. Notably, the tandem composite recombinant S. cerevisiae vaccine strain integrating p30-Fcγ and p54-Fcα gene fragments of two expression vectors in proper order is supposed to express P30-Fcγ and P54-Fcα fusion proteins simultaneously. Sequence analysis demonstrated that p30-Fcγ, and p54-Fcα fusion gene fragments conveyed by the expression plasmids have been successfully integrated into the genome of ST1814G and that the integrity of p30 and p54 were conserved and maintained stably without any mutations.
-
P30-Fcγ and P54-Fcα fusion proteins were fused to the cell surface binding factor Aga2 and expected to be displayed on the surface of ST1814G by linking to the Aga1 protein on the wall of yeast cells through a disulfide bond (Fig. 1B). The newly established anchored-yeast cell wall structure mentioned above carries a His tag to allow Western blotting, immunofluorescence and flow cytometry analysis staining intentionally.
As can be observed in Fig. 2A, Western blot analysis showed that no specific P30-Fcγ and P54-Fcα fusion proteins bands were detected in both the supernatant or the precipitated lysate of the negative control group, while specific bands of a molecular weight of approximately 30 kDa were detected in the lysates of the three recombinant S. cerevisiae strains after incubating anti-His antibody, indicating that P30-Fcγ and P54-Fcα fusion proteins were efficiently expressed by recombinant S. cerevisiae as expected. The other bands near the target band might be the results of glycosylation or the formation of a dimer of these two fusion proteins according the online glycosylation prediction. Especially, the protein bands of three recombinant S. cerevisiae strains present a deeper imprint after pretreatment of β-mercaptoethanol, which is capable of destroying the disulfide bonds between Aga1 and Aga2 to elute more fusion proteins attached to the cell wall of S. cerevisiae.
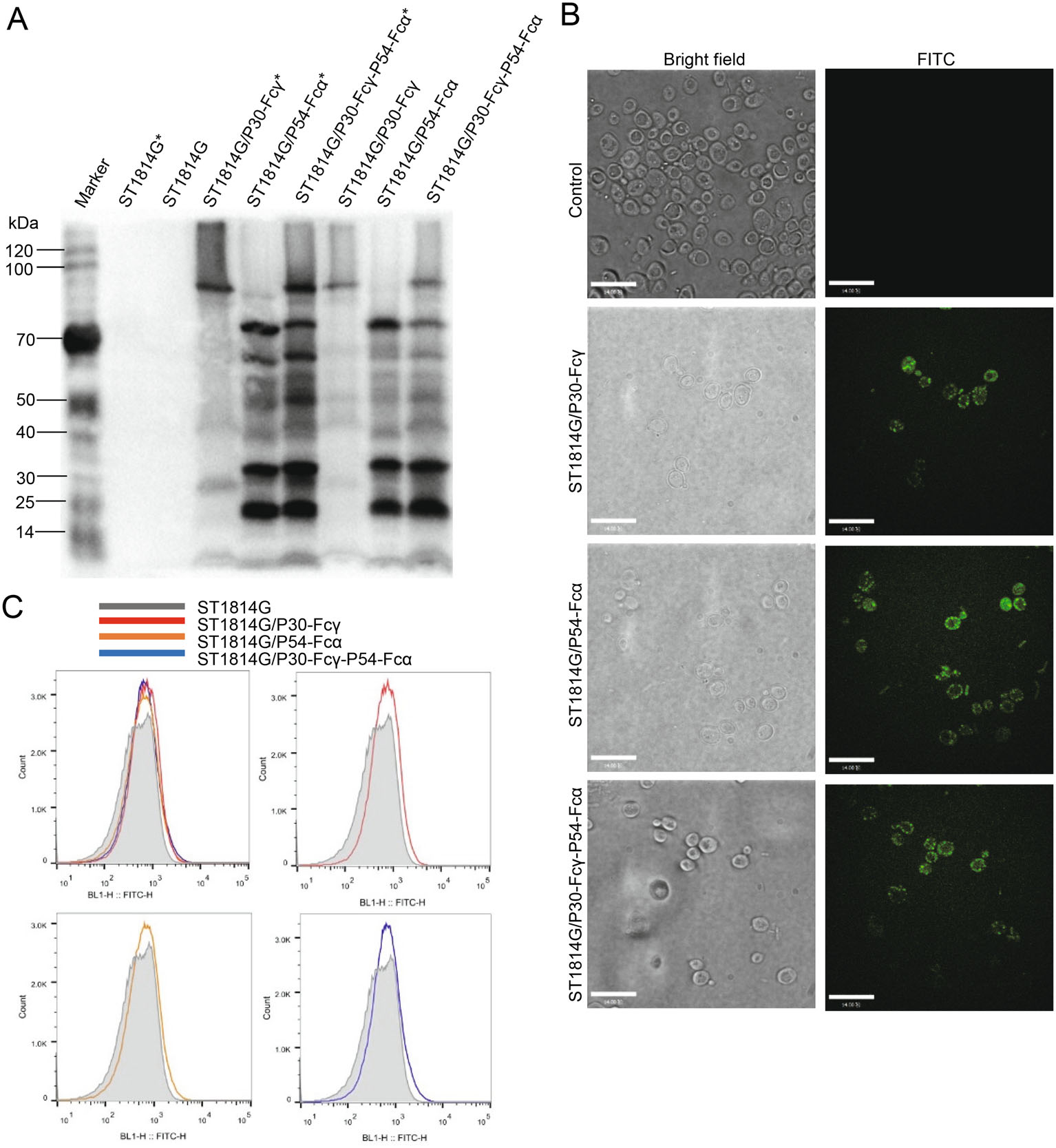
Figure 2. Surface displaying of P30-Fcγ and P54-Fcα fusion proteins on S. cerevisiae. A ST1814G (negative control), ST1814G/P30-Fcγ, ST1814G/P54-Fcα, ST1814G/P30-Fcγ-P54-Fcα were collected after cultivation for 24 h. The precipitated lysate were subjected to Western blot analysis. ST1814G, ST1814G/P30-Fcγ, ST1814G/P54-Fcα, ST1814G/P30-Fcγ-P54-Fcα with a normal sample preparation, while ST1814G*, ST1814G/P30-Fcγ*, ST1814G/P54-Fcα*, ST1814G/P30-Fcγ-P54-Fcα* were treated with β-mercaptoethanol to reduce the disulfide bonds. B ST1814G (negative control), ST1814G/P30-Fcγ, ST1814G/P54-Fcα, ST1814G/P30-Fcγ-P54-Fcα were collected after cultivation for 24 h. Yeast cells were fixed on glass slides and the presence of ASFV proteins were analyzed by confocal microscopy. The anti-His mouse antibody was used as the primary antibody. FITC-conjugated anti-mouse IgG was added as secondary antibody for immunofluorescence microscopy analysis. C Expression profiles of recombinant proteins were also determined by flow cytometry. Gray lines (dark shades) represent control cells, while positive cells are indicated by lines of different colors. Red, orange, blue lines stands for ST1814G/P30-Fcγ, ST1814G/P54-Fcα, ST1814G/P30-Fcγ-P54-Fcα, respectively.
As be identified in Fig. 2B, immunofluorescence microscopy results showed that no green fluorescence was detected in the negative control, whereas specific green fluorescence was detected in three experimental groups. P30-Fcγ and P54-Fcα fusion proteins were seen as bulk accumulation of green fluorescent protein (GFP) surrounded the surface of the three recombinant S. cerevisiae strains, indicating that antigenic proteins P30-Fcγ and P54-Fcα were expressed on the cell surface of the recombinant S. cerevisiae strains as expected.
Similar to the results above, flow cytometry analysis results in Fig. 2C suggest that compared with the negative control group (Red line), the curves' peak of the three recombinant S. cerevisiae strains showed a significant apparent shift to the right (Lines in different colors), demonstrating that P30-Fcγ and P54-Fcα fusion proteins were located on the surface of the recombinant S. cerevisiae cells.
Taken together, these data suggested that P30-Fcγ and P54-Fcα fusion proteins expressed and located on the surface of three recombinant S. cerevisiae strains successfully.
-
The growth of the fermentation kinetics of the three recombinant S. cerevisiae strains were investigated to access the impact of P30-Fcγ or P54-Fcα foreign proteins. The mRNA expression level of p30 gene of the single expression S. cerevisiae strain ST1814G/P30-Fcγ gradually increased, reaching the peak at 24 h, while the mRNA expression level of p54 gene of the tandem composite expression S. cerevisiae strain ST1814G/P30-Fcγ-P54-Fcα reached the peak at 36 h, as displayed in Fig. 3A.
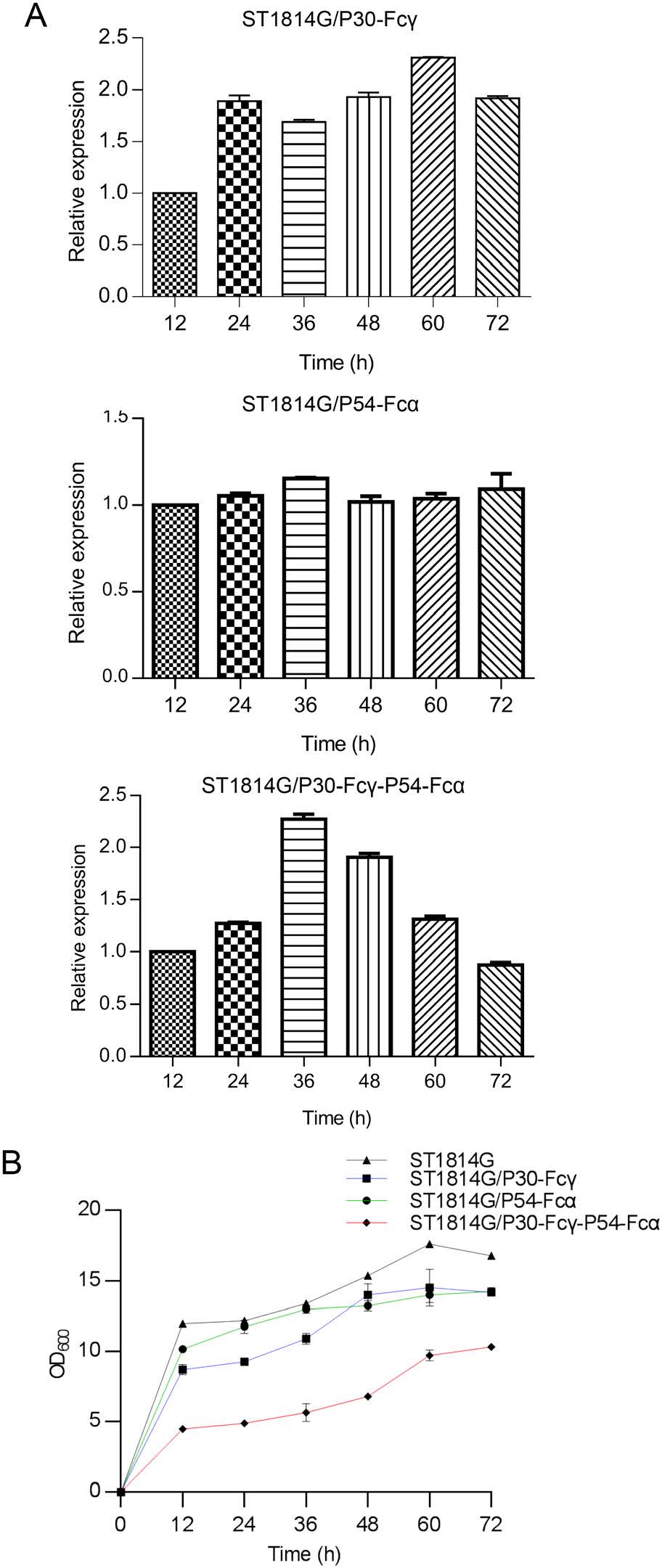
Figure 3. The fermentation kinetics of the recombinant S. cerevisiae strains. A The mRNA expression levels of p30 and p54 genes in the recombinant S. cerevisiae strains were quantified by qPCR. The ACT1 gene was used as internal control. B The growth curve of the three recombinant S. cerevisiae strains. The liquid OD600 nm value of the three recombinant S. cerevisiae strains cultures at the cultivation time points of 12 h, 24 h, 36 h, 48 h, 60 h, 72 h is determined by Spectrophotometer. The host S. cerevisiae strain ST1814G were set as the blank control.
For the purpose of measuring the relationship of yeast cells growth and proteins expression, the comparative growth curves between the host S. cerevisiae strain and the three recombinant S. cerevisiae strains were performed. The growth curve of the two single expression recombinant S. cerevisiae strains ST1814G/P30-Fcγ and ST1814G/P54-Fcα was similar to that of the host S. cerevisiae strain ST1814G shown in Fig. 3B. In detail, 0–12 h was the logarithmic growth phase while the amount of yeast cells reached the stable stage after 24 h. The peak of the amount of yeast cells appeared at the time point of 60 h. However, the amount of the tandem composite recombinant S. cerevisiae vaccine strain ST1814G/P30-Fcγ-P54-Fcα increased slowly and reached the vertex after cultivation for 72 h. Notably the maximum amount of yeast cells of ST1814G/P30-Fcγ-P54-Fcα was apparently lower than the host S. cerevisiae strain ST1814G and the two single expression recombination strains, indicating simultaneous expression of P30 and P54 might be a burden for recombinant S. cerevisiae strains. In conclusion, the growth of the three recombinant S. cerevisiae strains were affected by the co-expression of P30 and P54 proteins, suggesting that the growth environment of S. cerevisiae needs to be optimized and improved.
-
Fc receptors exist on the surface of IPEC-J2 cells and mature macrophages, which are responsible for recognizing and modulating the particles, thus prompting IPEC-J2 and PAM cells to phagocytose exogenous particles. Fcγ and Fcα receptors expressed on the surface of IPEC-J2 and PAM cells as expected (Fig. 4A, Supplementary figure S2). The mRNA expression levels of FcγRI and FcαRI on PAM cells reached a peak at 12 h, while that of FcγRI on IPEC-J2 cells reached a peak at 24 h. IPEC-J2 and PAM cells were incubated respectively with three recombinant S. cerevisiae strains subsequently.
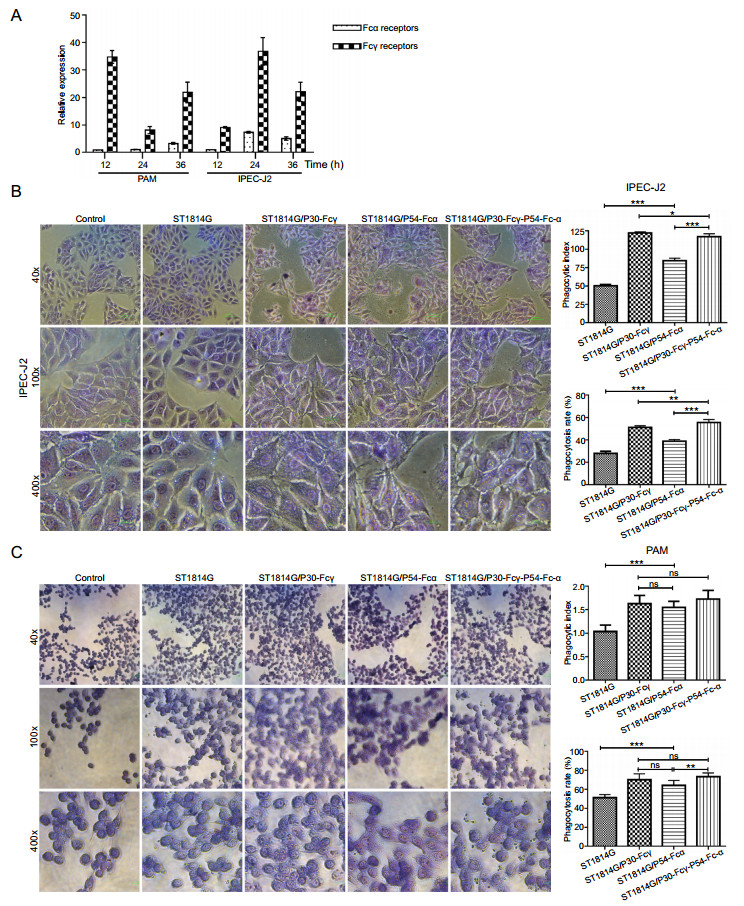
Figure 4. Fc fragment prompt binding and phagocytosis of the recombinant S. cerevisiae strains by porcine cells. A The mRNA expression levels of Fcγ receptor and Fcα receptor of PAM and IPEC-J2 cells were quantified by qRT-PCRb-Actin gene was used as internal control. B, C The adsorption phagocytosis to the host S. cerevisiae strain and three recombinant S. cerevisiae strains of IPEC-J2 cells (B) and PAM cells (C) under light microscopy. PAM cells were divided into blank control group, the host S. cerevisiae strain control group and three experimental groups. ST1814G, ST1814G/P30-Fcγ, ST1814G/P54-Fcα, ST1814G/P30-Fcγ-P54-Fcα were added into the corresponding chamber in the six-well plate. The phagocytosis process was observed under a microscope, and the phagocytic rate and phagocytic index were detected. The phagocytosis rate (%) = Number of macrophages engulfing yeast cells in 200 macrophages/200; the phagocytic index (%) = Total number of yeast cells engulfed by 200 macrophages/200. *P < 0.05; **P < 0.01; and ***P < 0.001.
As shown in Fig. 4B and 4C, three recombinant S. cerevisiae strains all adhered well to the surface of IPEC-J2 cells and were phagocytize by PAM cells. Compared with the host S. cerevisiae strain group, the percentages of phagocytic positive cells and the phagocytic indexs of IPEC-J2 and PAM cells both showed a significant upward trend. In addition, a larger quantity of quantity of S. cerevisiae particles which expressed P54-Fcα fusion protein adhered to IPEC-J2 cells, demonstrating that the expression of Fcα significantly increased the adhesion effect of S. cerevisiae on IPEC-J2 cells. In total, the expression of two Fc-fused ASFV proteins proved that Fc fragment were necessary to enhance the adhesion and phagocytation of recombinant S. cerevisiae cells to intestinal cells and PAM cells. Notably the adsorption and phagocytic ability to the tandem complex expression S. cerevisiae strain was significantly higher than basic level, indicating that Fcγ and Fcα mediated the binding and devouring of Fc fused-ASFV antigen proteins to IPEC-J2 and PAM cells jointly, and synergy effects were observed.
-
To test whether the three recombinant S. cerevisiae strains expressing ASFV antigens elicit a strong humoral response, we collected pig sera and analyzed P30/P54-specific IgG and IgA after oral-immunization with the S. cerevisiae-vectored recombinant P30 and P54 antigens. P30, P54, K145R-specific IgG and IgA antibodies in sera were evaluated by ELISA from pigs of the oral-vaccinated group and the control group. The prokaryotic expression vectors transformed into E. coli BL21(DE3) were used to generate affinity-purified recombinant ASFV proteins P30, P54 or K145R (Supplementary figure S3), which were used for detection of specific antibody production by ELISA. Pigs had few detectable anti-P30/P54 specific antibodies before immunization. Compared with control pigs immunized with PBS, all of the pigs immunized with the three recombinant S. cerevisiae strains developed P30/P54-specific IgG/IgA antibodies after the second or third oralimmunization. Statistical analysis (shown in Fig. 5) showed that pigs immunized with recombinant strains present high levels of P30/P54-specific antibodies in all pigs throughout the test cycle. Pigs that were immunized three times had higher P30/P54-specific IgG antibodies levels than pigs immunized two times. In addition, higher levels of IgA antibody production in response to P30 and P54 were observed in pigs immunized with three recombinant S. cerevisiae strains compared to PBS control. The K145R-specific IgG/IgA antibodies indicating the level of natural ASFV infection remained undetectable change, which demonstrated that control pigs immunized with PBS and the pigs immunized with the three recombinant S. cerevisiae strains faced same survival challenge throughout the experimental process. In short, the results showed that vaccination with three recombinant S. cerevisiae strains expressing ASFV antigens induced both humoral and ASFV-specific strong antibody responses to all the vaccine candidate antigens in swine. Our studies had confirmed that the synthetic genes p30, p54 used to generate the S. cerevisiae-vectored ASFV constructs could express authentic antigens. However, whether the primed antibodies can neutralize ASFV remains unknown.
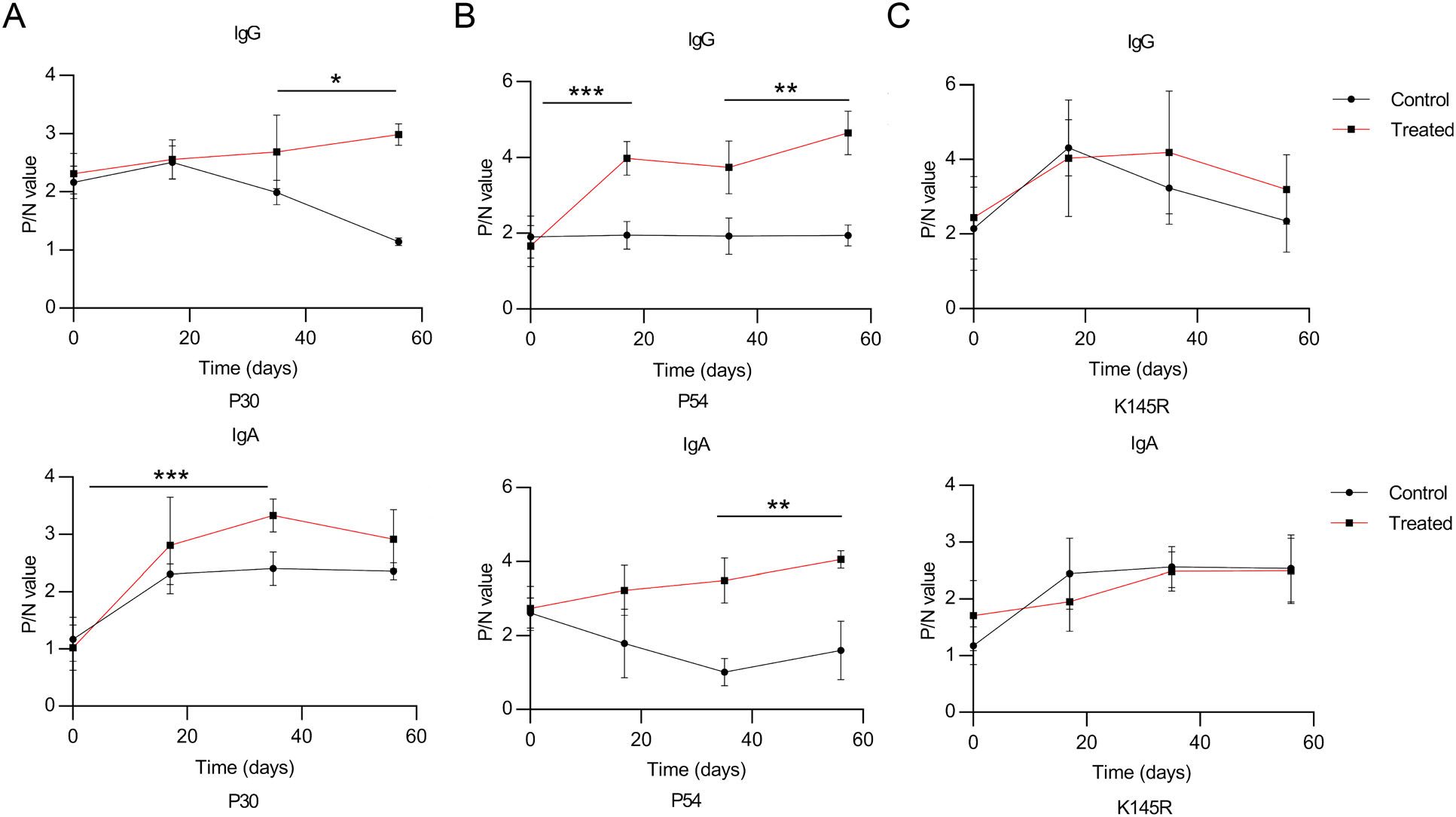
Figure 5. The recombinant S. cerevisiae strains stimulate specific antibody responses in vaccinated pigs. Vaccinated pigs were fed with the recombinant S. cerevisiae strains three times at 2-week interval. The titers of P30/P54/K145R-specific total IgG and IgA antibodies in serum were evaluated by ELISA. Serum samples were collected after the 1st, 2nd, and 3rd immunization. The individual animal response to each antigen was evaluated in triplicate and is depicted as the mean of the absorbance values at 450 nm minus the mean absorbance of cognate pre-immunization serum. The antigenspecific titers among the treatment groups were compared using ANOVA, followed by Tukey's multiple-comparison test. Error bars show the standard deviation between triplicates. The mean and standard error of antibodies of each group are shown. *P < 0.05; **P < 0.01; and ***P < 0.001.
Construction of p30-Fcγ and p54-Fcα Expression Vectors and Screening of the Recombinant S. cerevisiae Strains Expressing ASFV Antigens
Surface Displaying of P30-Fcγ and P54-Fcα Fusion Proteins on S. cerevisiae
The Fermentation Kinetics of the Recombinant S. cerevisiae Strains
Fc Fragment Prompt Binding and Phagocytosis of the Recombinant S. cerevisiae Strains in Porcine Cells
The Recombinant S. cerevisiae Strains Stimulate Specific Antibody in Oral Immunization of Pigs
-
ASFV has extremely complex viral genome, and the function of the virus-encoded proteins in the mechanism of virion assembly, proliferation, and immune protection remains unclear, which seriously blocks the development of strategies for the prevention and vaccines (Colgrove et al. 1969). Since the digestive tract and respiratory tract are the two main infection routes of ASFV, mucosal immunity might be the first line of defense against ASFV. So, it is rational and feasible to develop subunit oral vaccines against ASFV. Because the mechanism of protein folding and secretion of S. cerevisiae is very similar to that of eukaryotic cells, it is more likely to correctly express and display viral antigen proteins than prokaryotic cells (Liu et al. 2011). Therefore, subunit vaccines can be developed by expressing specific antigenic proteins based on the S. cerevisiae surface display system, an effective antigen delivery platform in vivo which might be capable of priming and expanding protective immunity in a manner similar to attenuated ASFV.
The multiplication and protein expression kinetics of recombinant yeast strains are crucial in vaccine production. On the one hand, the increasing metabolic pressure of recombinant yeast expressing exogenous genes and the lytic proteins produced by high-density culture of yeast inhibit the regular growth of them. Compared to the host strain ST1814G and two single-expression recombinant S. cerevisiae strains constructed in our study, the total cell mass of the tandem-composite-expression recombinant S. cerevisiae strain (express P30-Fcγ and P54-Fcα fusion proteins) was significantly lower (Fig. 3C). There is no doubt that more considerable re-optimization of yeast culture conditions was required by adjusting multiple factors such as pH, oxygen concentration, and carbon source feed rate. On the other hand, co-expression of multiple proteins involves a series of elements, such as the order of genes, position effects, the type and number of promoters, and the presence or absence of terminators, which demands more in-depth exploration and research.
The design of subunit vaccines based on two or more ASFV antigens have generated different protective outcomes, demonstrating that these antigens do play some role in host protection (Doytchinova and Flower 2007). Identification of ASFV targets that play a role in protection is rather important for the development of a highly efficacious subunit vaccine against ASFV. Still, it is not completely clear which ones play a significant role in protection against ASFV, although several immunogenic ASFV targets have been identified. Our study screened for potential antigens stimulating protective humoral immunity and cellular immunity based on existing ASFV vaccine research progress, and selected p30 and p54 as the candidate genes in vaccine design finally. Previous studies have shown that antibodies against P72 (Woźniakowski et al. 2017) and P54 (Gallardo et al. 2009) enable to inhibit the first step of the viral replication cycle associated with viral attachment, while anti-P30 antibodies involve in the second step associated with viral internalization (Sanna et al. 2017). This could be explained by the two neutralization mechanisms observed in serum of convalescent pigs, suggesting that specific ASFV proteins serve important roles in different neutralization mechanisms (Lubisi et al. 2005). We developed three recombinant S. cerevisiae strains expressing P30 and P54 antigens to achieve protection through subunit vaccines using a cocktail of ASFV antigens. Western blot analysis (Fig. 2A) of cell lysates from three recombinant S. cerevisiae strains confirmed the successful expression of ASFV protein P30/P54. Nevertheless, the concentration of ASFV proteins displayed on the surface of S. cerevisiae cells is considerably low by speculating the amount of visible green fluorescence in the microscope field (Fig. 2B), indicating that the expression of ASFV proteins in recombinant S. cerevisiae strains need to be further optimized through performing codon optimization, employing efficient promoters and discovering suitable chromosome integration sites.
The Fc receptor-targeted mucosal immunity was reported to effectively induce protective immunity and maximize the immune effect against various mucosal pathogens (Czajkowsky et al. 2012). In addition, fusion expression of recombinant protein and human IgG antibody Fc segment is one of the important strategies to obtain long-acting recombinant proteins preparation by increasing the stability in vivo (Jazayeri and Carroll 2008). Therefore, we expressed P30/P54-Fcγ/Fcα fusion proteins and develop an Fc receptor-mediated oral vaccine against ASFV. The result showed that IPEC-J2 and PAM cells exhibit greater phagocytic capacity to the three recombinant S. cerevisiae strains (Fig. 4B, 4C), indicating that P30-Fcγ and P54-Fcα fusion proteins promoted the phagocytosis of recombinant S. cerevisiae cells as antigens by binding to Fc receptors on the surface of IPEC-J2 and PAM cells. It can be inferred that Fcα chain held the superiority of promoting adhesion, while Fcγ chain possessed the ability to promote phagocytosis. Overall, Fc fusion-expressed recombinant proteins might promote the process of binding and endocytosing antigens of porcine-derived cells, thus accelerating antigen presentation and improving immune efficiency.
In conclusion, we developed ASFV oral vaccines based on the S. cerevisiae surface display system for the first time and evaluated the immune efficacy of three recombinant S. cerevisiae strains. The expression of S. cerevisiae-vectored ASFV antigens was confirmed and specific antibodies against ASFV were detectable by ELISA in oral-immunized pigs. Robust immune responses in swine was induced by using a cocktail of S. cerevisiae-vectored ASFV antigens. We observed higher levels of P30/P54-specific IgG and IgA antibodies in all pigs which were fed three recombinant S. cerevisiae strains expressed ASFV P30/P54-Ig heavy chain fusion proteins, and the antibodies titers continued to increase in response to the second immunization (Fig. 5), suggesting that it may be possible to improve the immune response to P30 and P54 antigens by providing multiple consecutive immunizations or by boosting with a heterologous system to optimize the induction of specific antibodies. Yet, the strategy has not been tested against virus challenge. Collectively, our research validated a novel S. cerevisiae-vectored approach to generate and deliver a cocktail of ASFV vaccine and proved that three recombinant S. cerevisiae strains elicited ASFV-specific antibody production, providing a rational strategy for further screening of ASFV antigen targets toward development of a multiantigen, efficacious vaccine for the prevention of ASFV infection. Further studies will be required to identify more immunogenic ASFV proteins and optimize the fermentation conditions of the recombinant S. cerevisiae strains to improve protein expression efficiency and the actual efficacy of vaccines.
-
This work was supported by the National Key Research and Development Program of China (2018YFD0500500).
-
JH conceived and designed the experiments. CC, DH, JS, Z T, LZ, M Z and KT performed the experiments. CC analyzed the data. JH contributed reagents/materials/analysis tools. CC and JH wrote the paper.
-
The authors declare that they have no conflict of interest.
-
The whole study was approved by the Administrative Committee on Animal Welfare of the Institute of Radiation Medicine, Academy of Medical Sciences, China (Laboratory Animal Ethical and Welfare Committee, permit number IRMDWLL-2019020). All institutional and national guidelines for the care and use of laboratory animals were followed.


















 DownLoad:
DownLoad: