HTML
-
Dengue virus (DENV) is one of the most important arthropod-borne viral pathogens worldwide with 390 million people at risk annually from tropic to subtropical regions, particularly in South-east Asia and the Pacific (Bhatt et al. 2013). Approximately 50 to 100 million dengue cases and 25, 000 deaths are estimated to occur each year (Bhatt et al. 2013; Murray et al. 2013). DENV infections can be asymptomatic or manifest as various clinical features, ranging from atypical non-severe or nonspecific febrile illness to potentially life-threatening dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS), depending on the infection status (Yung et al. 2015; Cucunawangsih and Lugito 2017). DENV belongs to the genus Flavivirus and comprises four distinct serotypes (DENV-1, DENV-2, DENV-3 and DENV-4) (Halstead 2003; Gould and Solomon 2008; Cox et al. 2012; Mustafa et al. 2015). As a member of Flaviviridae family, the virion is an enveloped particle containing a positive singlestranded RNA of ~ 11 kb. The viral RNA genome is translated into a single polyprotein that is cleaved by cellular and viral proteases into three structural proteins: capsid protein (C); membrane protein (M); envelope protein (E), and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) (Khetarpal and Khanna 2016). The C protein is complexed with the viral RNA genome to form the viral nucleocapsid, which is surrounded by a host cell-derived lipid bilayer (Rodenhuis-Zybert et al. 2010). DENV genome encapsidation is a crucial stage in viral replication and assembly, involving both host factors and DENV proteins. To-date, Dengvaxia, a dengue vaccine is only approved for people ages 9 through 45 years old with laboratory-confirmed previous dengue infection and living in endemic areas (Aguiar et al. 2016). No other potent vaccines or antiviral drugs are available for DENV infection, in part due to the limited understanding of key DENV-host interactions.
Recent progress has enhanced our knowledge of how viruses coopt host cell processes to favor their own replication cycles. For example, host lipid metabolic pathways are hijacked by a multitude of viruses to complete their replication and assembly. As a class of bioactive molecules, lipids are a key cellular metabolic energy source that are synthesized and utilized through different pathways. Most neutral lipids are stored in lipid droplets (LDs) which are the endoplasmic reticulum (ER)-derived intracellular organelles consisting of a hydrophobic core and a surrounding phospholipid monolayer (Walther and Farese 2012; Lin et al. 2014; Bersuker and Olzmann 2017; Zhang et al. 2017). LDs are complex and dynamic organelles that mediate lipid metabolism, membrane trafficking, membrane biosynthesis, signal transduction and even immune defenses (Ding et al. 2013; Wang 2016). DENV and hepatitis C virus (HCV) enhance LDs formation for use as platforms for viral assembly (Barba et al. 1997; Samsa et al. 2009; Zhang et al. 2017). DENV-capsid and HCV-core are recruited to the LDs to facilitate virus encapsi-dation, and pharmacological inhibition of fatty acid synthase (FASN) using C75 significantly impairs DENV replication and morphogenesis (Samsa et al. 2009; Poh et al. 2012). Interestingly, recent studies demonstrate that DENV infection triggers lipophagy to deplete LDs for cellular β-oxidation, suggesting that DENV may manipulate cellular lipid metabolism to favor its replication (Heaton et al. 2010; Zhang et al. 2018). The roles of LDs during DENV infection therefore requires further clarification.
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is an important tumor suppressor that is frequently mutated or down-regulated in human cancers. It is a dual-specificity phosphatase with both lipid and protein phosphatase activity that antagonizes phosphoinositide 3-kinase (PI3K) to regulate cell growth and survival (Song et al. 2012). In addition to its potent anti-tumor effects, recent studies have expanded its roles during lipid metabolism. Previous studies demonstrate that HCV downreg-ulates PTEN and the accumulation of large LDs, and that HCV secretion is regulated by the protein phosphatase activity of PTEN through the modulation of cellular cholesterol metabolism (Peyrou et al. 2013). Alterations in PTEN expression during HCV infection stimulate lipoge-nesis by modulating microsomal triglyceride transfer protein (MTP) and/or sterol regulatory element binding proteins (SREBPs) activity (Domitrovich et al. 2005; Waris et al. 2007). Deregulation of PTEN is associated with a spectrum of metabolic disorders related to hepatic injury. Considering that the formation of large LDs and the induction of lipogenesis could be two separate distinct events, the role of PTEN during virus infection therefore needs to be further elucidated.
DENV infection seems to cause some hepatotoxic effects. Although most cases are asymptomatic, including mild elevations in serum bilirubin or elevated transaminases, acute liver failure (ALF) may occasionally worsen clinical status (Samanta and Sharma 2015; Dalugama and Gawarammana 2018). In this study, we focused on whether the regulation of PTEN during lipid metabolism and LDs formation affects the DENV lifecycle in infected hepato-carcinoma cells. We reveal alterations in PTEN expression and lipid phosphatase activity during DENV infection that leads to decreased LDs formation and FASN expression. This expands our knowledge of the DENV-PTEN interactions required for virus replication and assembly.
-
Huh7, HEK 293T and Vero cells were grown in complete Dulbecco's Modified Eagle's Medium (DMEM) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Gibco BRL, USA) supplemented with 100 nmol/L nonessential amino acids (NEAA), 1 mmol/L L-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin at 37 ℃ in 5% CO2. Aedes albopictus mosquito (C6/36) cells (kindly provided by Prof. Jing An from Capital Medical University, Beijing, China) were cultured in RPMI-1640 medium (Gibco, Invitrogen, USA) supplemented with 10% FBS and antibiotics at 28 ℃.
DENV-2 (strain Tr1751, kindly provided by Prof. Jing An from Capital Medical University, Beijing, China) virus was propagated in C6/36 cells, and virus stocks were stored at — 70 ℃ until use. Viral titers were detected by plaque assay and are shown as plaque-forming units (PFU) per mL.
-
Polyclonal rabbit anti-DENV capsid and NS3 antibodies were obtained from GeneTex (Irvine, CA, USA). Polyclonal rabbit antibodies to Akt, phospho-Akt-Ser473, phospho-Akt-Thr308 and FoxO1 were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies to Acetyl Coenzyme A Carboxylase (ACC), Fatty Acid Synthase (FASN) and Maf1 were purchased from Abcam (Cambridge, MA, USA). Monoclonal rabbit anti-PTEN antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Beyotime (Hangzhou, China). Monoclonal mouse anti-GAPDH antibodies were purchased from Abmart (Shanghai, China). Polyclonal rabbit anti-LC3B antibodies and protease inhibitor cocktail were purchased from Sigma (St. Louis, MO, USA). Lipofectamine and Alexa-fluor 488 secondary antibodies were purchased from Invitrogen (Shanghai, China).
-
pLenti-PTEN and GV248-shPTEN (short hairpin RNAs targeting PTEN) were used to express wild type (wt) and silenced PTEN in cells, respectively (Gao et al. 2015). Single amino acid substitutions (PTEN Y138L, G129E or C124S) were introduced into the pLenti-PTEN plasmid via QuickChange Lightning Site-Directed Mutagenesis Kit (Stratagene) as previously described (Qin et al. 2013). The desired mutations were confirmed by nucleotide sequencing by RuiDi (Shanghai, China).
-
Huh7 cells were infected with DENV-2 at a multiplicity of infection (MOI) of 1 or the indicated MOIs for 2 h in serum-free optiMEM. Cells were washed with PBS and cultured in fresh complete medium for various times until harvesting.
-
Lentiviral particles pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G) were generated in HEK 293T cells through co-transfection of lentiviral plasmids encoding wt PTEN, PTEN mutants (PTEN Y138L, G129E or C124S), PTEN shRNA or lentiviral vectors with plasmids encoding compatible packaging proteins and VSV-G (Gao et al. 2015; Peng et al. 2018). Forty-eight hours posttransfection, cell supernatants were collected, filtrated, and used to transduce Huh7 cells. Empty lentiviral vector and lentiviral plasmid expressing scramble shRNA was used as lentivirus negative control (LNC) and shRNA negative control (SNC), respectively. The efficiency of PTEN overexpression or downregulation were confirmed by Western blot analysis using specific anti-PTEN antibodies. At 48 h post-transduction, cells were infected with DENV-2 at the indicated MOIs for 48 h, as described above.
-
Virus titers in viral stocks or culture supernatants were determined by plaque assay as previously described (Delbruck 1940; Peng et al. 2018). Briefly, Vero cells were infected with serially diluted virus stocks or supernatants from DENV-infected cells. After virus adsorption, cells were washed with pre-warmed PBS, and overlayed with medium containing 1.3% methylcellulose. Cells were incubated for 7 to 8 days at 37 ℃, and stained with 1% crystal violet. Plaques were counted and the viral titers were determined as PFU/mL.
-
Cells were lysed in ice-cold RIPA buffer (Beyotime, China) containing protease inhibitors as previously described (Gao et al. 2015; Qin et al. 2015). After centrifugation, protein concentrations were determined using the Bradford method (Beyotime, China). Proteins (40 μg) were separated by 12.5% (w/v) SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to PVDF membranes (Millipore, USA) using Trans-Blot apparatus (BioRad). Proteins of interest were detected with specific primary antibodies and corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies. Immunoreactivity was visualized by enhanced chemiluminescence using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific, CA, USA). Quantifications were performed using ImageJ software (National Institutes of Health) and adjusted for densitometry with corresponding loading controls (GAPDH).
-
Total RNAs were extracted using Trizol (Invitrogen) according to the manufacturer's instructions (Qin et al. 2004, 2007). Total RNA (1 μg) was reverse transcribed using M-MLV reverse transcriptase (Promega) with random hexamers. Quantitative real-time PCRs were performed using SYBR Green Master kits (TaKaRa) on a 7300Plus Real-Time PCR system (Thermo Fisher Scientific, CA, USA) and normalized to GAPDH. Primer sequences for each target gene were listed as Supplementary Table S1. All reactions were performed in triplicate.
-
Huh7 cells were transduced with lentiviruses expressing wt PTEN, PTEN mutants, PTEN shRNA or empty vector control for 48 h, and infected with DENV-2 at a MOI of 1 for a further 48 h. For the detection of LDs, cells were fixed with 4% formaldehyde, washed with PBS, and per-meabilized with 0.5% saponin in PBS. After being blocked in 3% bovine serum albumin (BSA), and stained with rabbit anti-DENV NS3 primary antibodies and Alexa Fluor 488 (goat anti-rabbit), cells were mounted with DAPI and Oil Red O to visualize cell nuclei and LDs, respectively (Kinkel et al. 2004). Images were obtained using a Zeiss laser-scanning contocal microscope and images were processed using Photoshop CS2 and Zeiss operating sottware. LDs were quantified according to area and number using ImageJ at a defined intensity threshold that was applied to all images.
-
Data represent the mean ± standard deviations (SD) of independent experiments. Results were analyzed using a Student's t test or one-way ANOVA using Prism software. Values were considered statistically significant when *P < 0.05, **P < 0.01 or ***P < 0.001.
Cells and Virus
Antibodies and Reagents
Plasmid Construction
Virus Infection
Lentiviral Particle Production and Target Cell Transduction
Plaque Assay
Western Blot Analysis
Real-Time PCR
Confocal Microscopy
Statistical Analysis
-
It has been reported that HCV intection induces a significant reduction in PTEN expression through 3'-UTR-mediated blockade of PTEN mRNA translation (Peyrou et al. 2013). As a member of Flaviviridae tamily, the effects of DENV on PTEN expression remain undefined. To explore the regulation of PTEN by DENV, we investigated its expression in Huh7 cells intected with DENV-2 (MOI: 1) by Western blotting and quantitative RT-PCR analysis. We found that DENV inhibited PTEN expression at protein level by 41% compared to mock cells (Fig. 1A, 1B). Quantitative RT-PCR results revealed no significant decrease in PTEN mRNA in DENV intected cells (Fig. 1C), suggesting post-transcriptional control of PTEN during DENV intection.
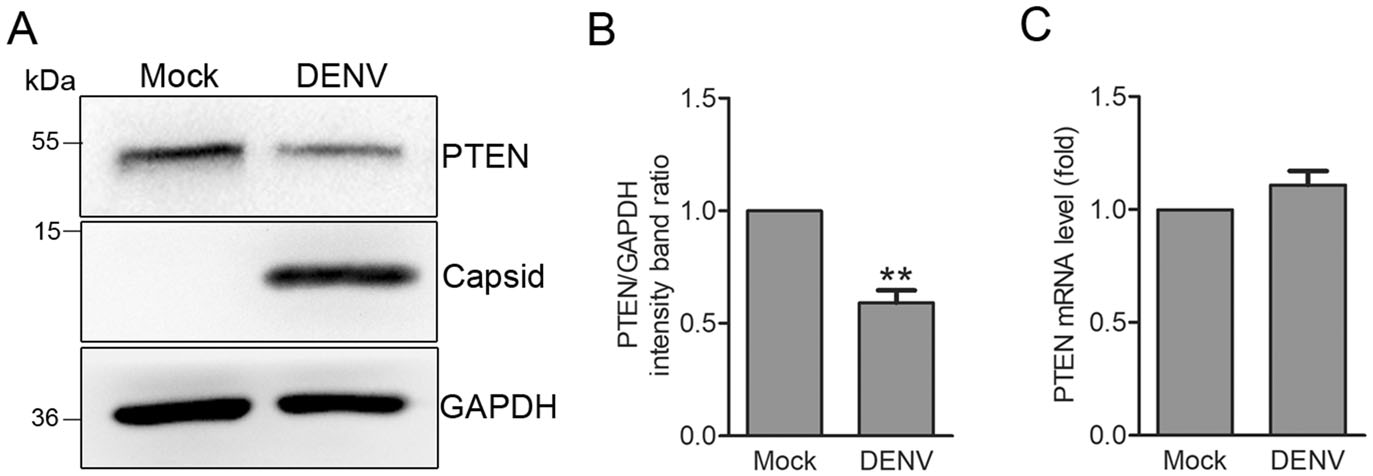
Figure 1. DENV infection decreases PTEN protein expression but not mRNA transcription. A Western blot analysis. PTEN expression was detected in Huh7 cells that were mock-treated or infected with DENV-2 at an MOI of 1. Cells were harvested 48 h post infection (hpi) and probed for anti-PTEN antibodies. GAPDH was used as a loading control. Representative blots (n = 3) are shown. B Band ratios of PTEN to GAPDH measured by densitometry. C Quantitative RTPCR analysis. Cells were harvested 48 hpi and PTEN mRNA levels were determined by quantitative RT-PCR. Graphs represent relative fold changes of PTEN mRNA to controls. Data are means of three independent experiments. Significance was analyzed using a Student's t test. **P < 0.01.
-
We next explored the effects of PTEN overexpression or silencing on the DENV replication cycle. Huh7 cells were transduced with lentiviruses expressing PTEN or PTEN shRNAs, and then intected with DENV-2 at an MOI of 1 for 48 h. PTEN overexpression and knockdown were confirmed by Western blot analysis. Overexpressing PTEN enhanced intracellular DENV-capsid expression while PTEN silencing had the opposite effects (Fig. 2A). Compared with control cells, the ratio of DENV capsid to GAPDH and intracellular DENV RNA levels increased by 70% and 86% in cells overexpressing PTEN measured by densitometry and quantitative RT-PCR, respectively (Fig. 2B, 2C). In contrast, blocking PTEN expression with the PTEN shRNA-expressing lentiviruses decreased DENV capsid expression and RNA levels by 71% and 66%, respectively (Fig. 2A-2C).
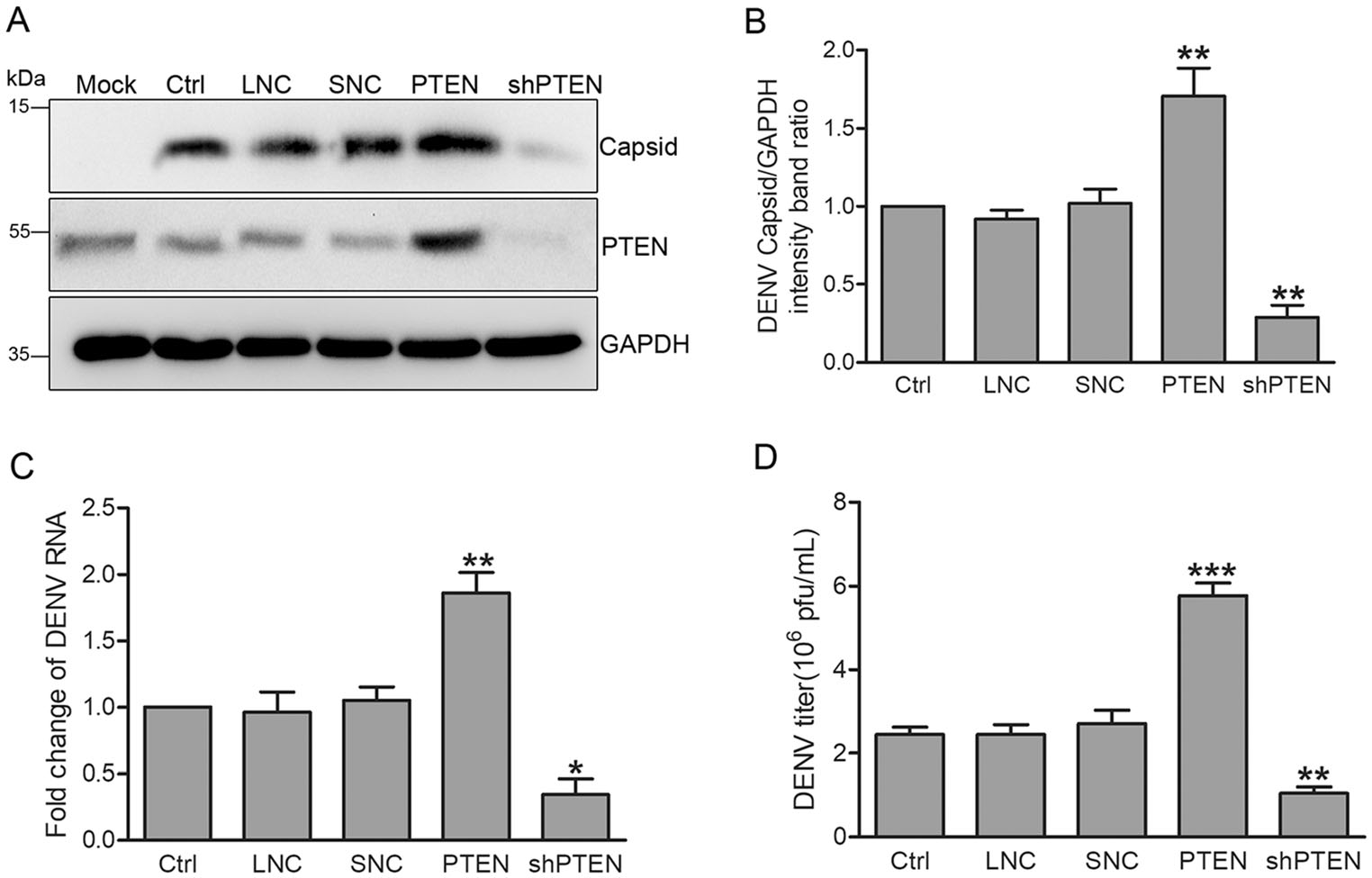
Figure 2. PTEN overexpression enhances DENV replication while PTEN silencing had the opposite effects. Huh7 cells were infected with lentiviruses expressing PTEN, PTEN shRNA, empty lentivirus vector and scramble shRNA. DENV-infected Huh7 cells were used as a positive control, while empty lentivirus vector and scramble shRNA treated cells were used as lentivirus negative control (LNC) and shRNA negative control (SNC), respectively. A Western blot analysis (n = 3) of cellular PTEN and DENV capsid expression. B Band intensities normalized to GAPDH. C Quantitative RT-PCR analysis of cellular DENV RNA levels using DENV capsid and GAPDH specific primers. Graphs represent relative fold changes of DENVRNA. D Supernatants were collected 48 hpi, and extracellular virus yields were determined by plaque assay in Vero cells and expressed as pfu/mL. Data are means of three independent experiments. Significance was analyzed using One-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001.
We next assessed whether modulating PTEN expression attects the production of intectious DENV progeny measured via plaque assays in Vero cells. The results showed that PTEN overexpression increased virus production by 136% while PTEN silencing decreased virion production by 57% (Fig. 2D).
Taken together, these data indicate that the etficiency of DENV replication and secretion correlate with PTEN expression, suggesting that PTEN overexpression enhances DENV replication.
-
To investigate whether the protein and/or lipid phosphatase activity of PTEN is required for DENV intection, we established Huh7 cells transduced with lentiviruses expressing mutant forms of PTEN, namely PTEN G129E (the lipid phosphatase inactive torm), Y138L (the protein phosphatase inactive form) and C124S (both the lipid and protein phosphatase inactive form) (Myers et al. 1997, 1998; Davidson et al. 2010). These transduced cells behaved as dominant negative mutants and reduced PTEN activity. Huh7 cells expressing the various forms of PTEN were infected with DENV-2 at an MOI of 1 for 48 h. As shown in Fig. 3, only wild type (wt) PTEN and the protein phosphatase deficient Y138L mutant enhanced intracellular DENV capsid expression (80% and 88% increase compared to control cells, respectively). Likewise, intracellular DENV RNA levels were approximately 78% and 89% higher than those of control cells, respectively (Fig. 3C). In contrast, PTEN G129E and C124S mutants did not affect DENV capsid expression or viral RNA levels (Fig. 3A-3C).
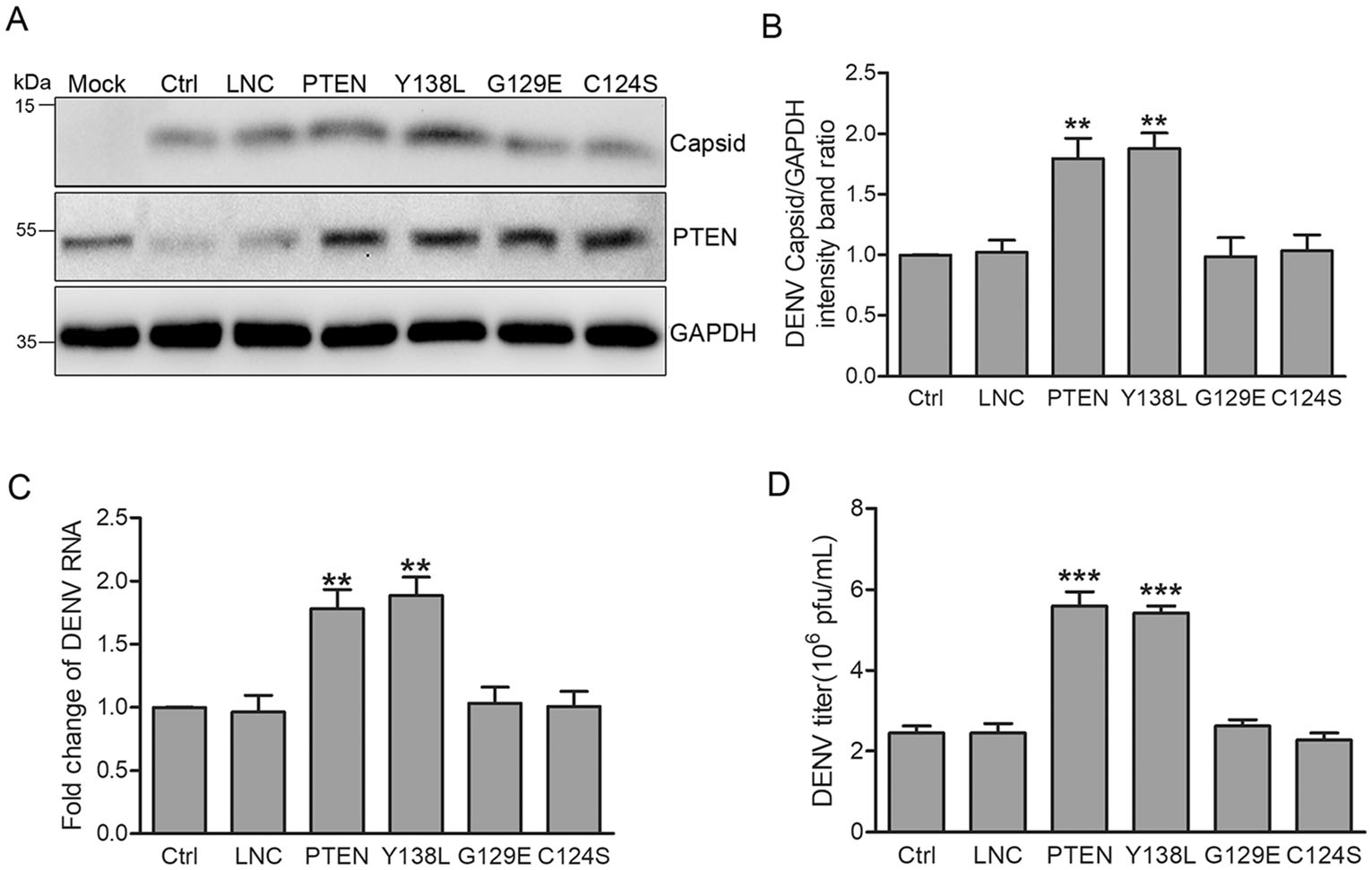
Figure 3. PTEN lipid phosphatase activity enhances DENV replication. Huh7 cells were transduced with lentiviruses expressing PTEN, PTEN mutants (Y138L, G129E, C124S) and empty lentivirus, respectively. Representative images are shown. A Western blot analysis of PTEN and DENV capsid expression. B Band intensities of DENV capsid to GAPDH. C Quantitative RT-PCR analysis of cellular DENV RNA levels. D Extracellular virus yields determined by plaque assay. Data are means of three independent experiments. Significance was analyzed with One-way ANOVA. **P < 0.01; ***P < 0.001.
Next, we performed plaque assays to examine the effects of PTEN mutants on the secretion of progeny virus particles. The expression of wt PTEN and protein phosphatase deficient Y138L mutant promoted DENV production (120% increase compared with control cells), while no changes were observed for PTEN G129E and C124S mutants (Fig. 3D).
These results indicated that only wt PTEN and the protein phosphatase deficient Y138L mutant promoted the expression of DENV capsid, intracellular viral RNA levels, and virion secretion. Thus, the lipid phosphatase activity of PTEN, but not its protein phosphatase activity, is required during DENV replication.
-
As a negative regulator of PI3K/AKT signaling, PTEN plays fundamental roles in cellular lipid metabolism in cancer pathogenesis (Menendez and Lupu 2007). Previous studies have shown that HCV core leads to the accumulation of large LDs through downregulating PTEN expression, while the loss of PTEN protein phosphatase activity promotes virus secretion by increasing LDs size and cholesterol ester (CE) content (Clement et al. 2011; Peyrou et al. 2013). DENV induces lipophagy to release free fatty acids (FFAs) for its replication (Heaton and Randall 2010), suggesting that lipid metabolism is also critical for DENV replication and infection. To determine whether the lipid phosphatase activity of PTEN regulates cellular lipid metabolism during DENV infection, we examined LDs formation using Oil Red O staining by confocal microscopy. Huh7 cells were transduced with lentiviruses expressing wt PTEN, PTEN mutants or PTEN shRNA and then infected with DENV-2 for 48 h. After fixation, cells were probed with DENV NS3 specific antibodies and stained with Oil Red O. Compared to mock-infected cells, the area and number of LDs per cell were reduced by 40% and 17% respectively following DENV-infection, which was also similar with those observed for lentiviral vector, PTEN G129E or C124S mutant transduced cells. However, the area and number of LDs per cell decreased by 80% (area) and 60% (number) in cells expressing wt PTEN and the PTEN Y138L mutant when compared with mock-infected cells. No significant changes in LDs area and number were observed in PTEN shRNA transduced cells (Fig. 4A, 4B). These data indicate that the PTEN Y138L mutant with lipid phosphatase activity retains wt PTEN ability to result in a reduction in LDs area and number following DENV-infection. This was consistent with previous studies demonstrating that DENV-induced lipophagy depletes LDs, releasing FFAs to undergo β-oxidation (Heaton and Randall 2010).
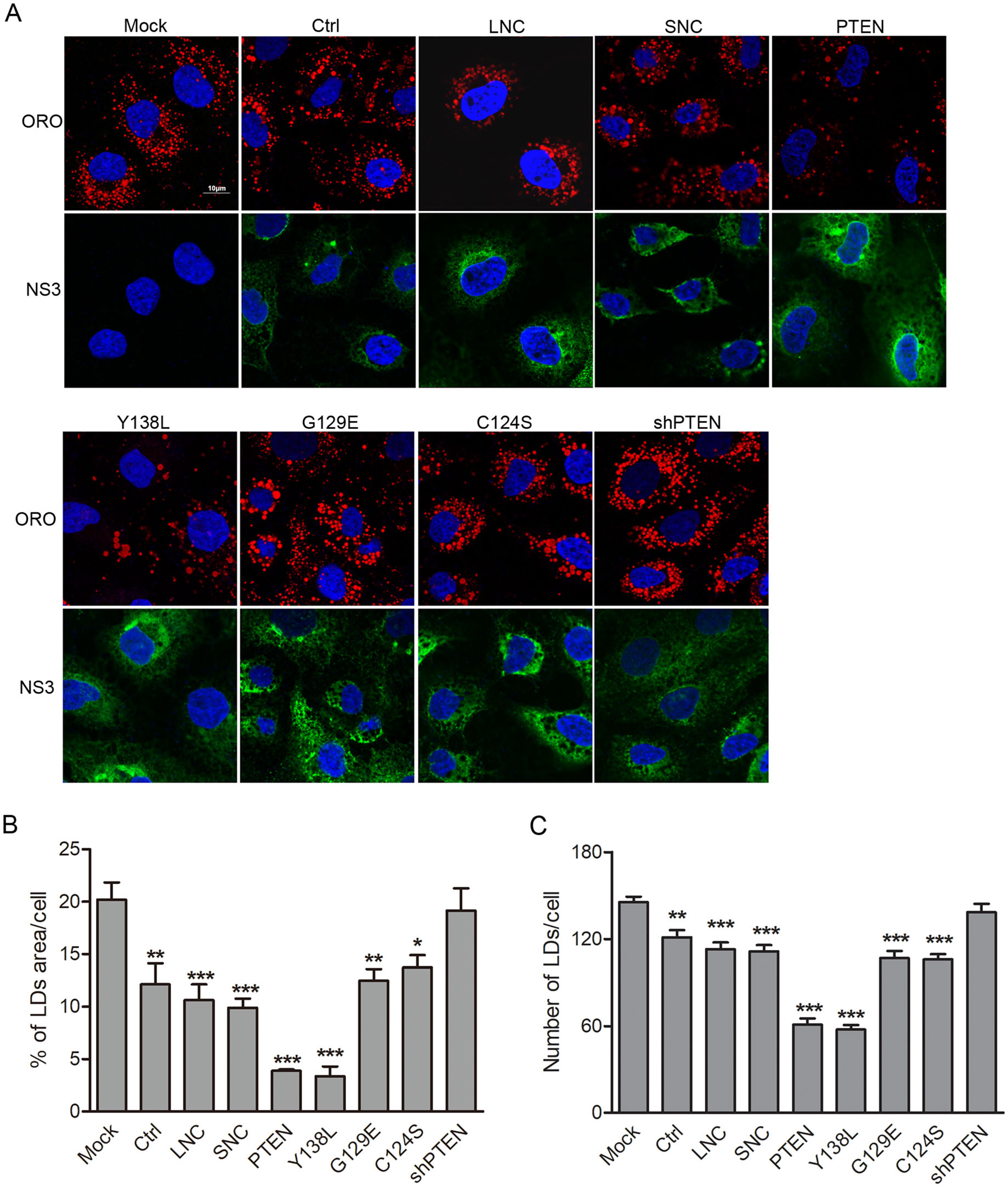
Figure 4. PTEN lipid phosphatase activity controls lipid droplet area and number in DENV-infected cells. Huh7 cells were transduced with lentiviruses expressing PTEN, PTEN mutants (Y138L, G129E, C124S), PTEN-shRNA, empty lentivirus and scramble shRNA, respectively, and then infected with DENV-2 for 48 h. A Cells were fixed, and stained with anti-DENV NS3 antibodies, DAPI and Oil Red O. Representative images are shown. Quantification of the total Oil Red O-positive area (B) and puncta (C) per cell using Image J. Data are presented as means from three independent experiments. Significance was analyzed using One-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001.
-
To explore the mechanism of PTEN in lipid metabolism in DENV-infected cells, we first examined the phosphorylation status of Akt as a representative downstream PTEN substrate. As shown in Fig. 5, compared with control cells, Akt phosphorylation at serine 473 (S473) and threonine 308 (T308) decreased in cells transduced with wt PTEN and the Y138L mutant, with the opposite phenotype observed in PTEN shRNA transduced cells. These results suggest that PTEN lipid phosphatase activity reduces PI3K activation, which inhibits Akt (Roh et al. 2010; Naderali et al. 2018).
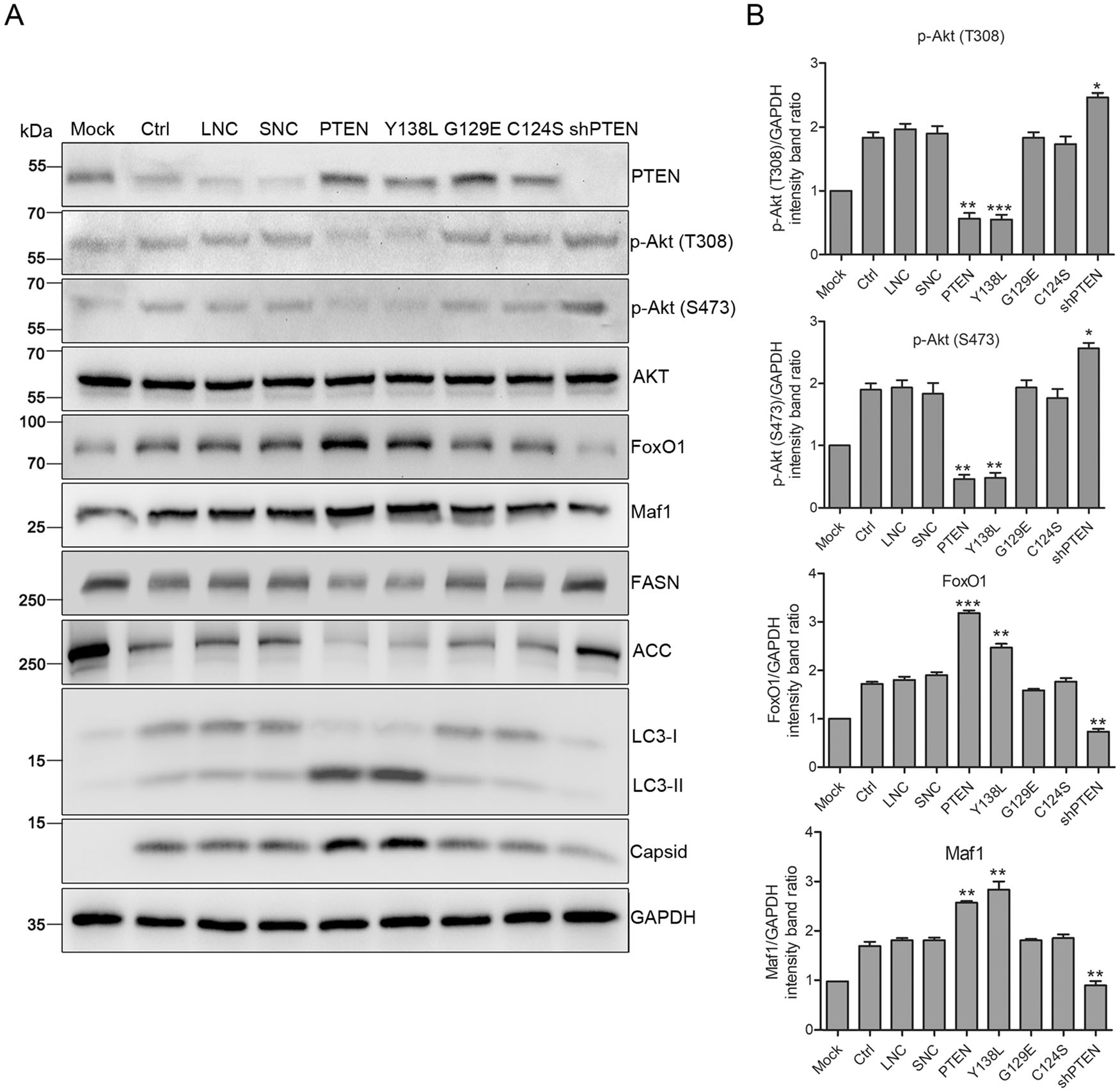
Figure 5. PTEN lipid phosphatase activity enhances DENV replication via Akt/FoxO1/Maf1 signaling and autophagy. Huh7 cells were transduced with lentiviruses expressing PTEN, PTEN mutants (Y138L, G129E, C124S), PTEN-shRNA, empty lentivirus and scramble shRNA, respectively, and infected with DENV-2 at MOI 1 for 48 h. A Samples were collected and subjected to western blot analysis. GAPDH was probed as a protein loading control. Representative blots are shown. B Band intensities of p-Akt (T308), p-Akt (S473), FoxO1 and Maf1 to GAPDH. Data are means of three independent experiments. Significance was analyzed using One-way ANOVA. *P < 0.5; **P < 0.01; ***P < 0.001.
FoxO1 is one of the major downstream targets of Akt that play an essential role in lipid metabolism (Johnson and Stiles 2016). The expression of FoxO1 increased in cells transduced with wt PTEN and Y138L mutant, but declined in cells transduced with PTEN shRNA (Fig. 5). Akt phosphorylates FoxO1 at three sites, hindering its nuclear localization (Johnson and Stiles 2016). Suppression of lipogenesis is mediated by the transcriptional function of FoxO1 through the regulation of two lipogenesis enzymes, FASN and Acetyl Coenzyme A Carboxylase (ACC), which is Akt dependent (He et al. 2010). In addition, FoxO1 can regulate Maf1, a crucial transcription factor related to lipid metabolism enzymes (Johnson et al. 2007; Deng et al. 2012). Maf1 expression was upregulated with increased FoxO1 expression in cells transduced with wt PTEN and the Y138L mutant, and downregulated in those transduced with PTEN shRNA. Correspondingly, upregulated Maf1 inhibited the expression of FASN and ACC in cells transduced with wt PTEN and the Y138L mutant, whilst the downregulation of Maf1 in cells transduced with PTEN shRNA also inhibited enzyme expression (Fig. 5). These results are consistent with previous studies demonstrating that Maf1 inhibition accelerates the expression of FASN and ACC and intracellular lipid accumulation, while overexpressing Maf1 blocked the lipogenesis (Palian et al. 2014).
Previous studies have shown that DENV-induced autophagy stimulates β-oxidation by depleting cellular LDs. Whether DENV-induced autophagy correlates with PTEN phosphatase activity was also investigated. As shown in Fig. 5, the conversion of LC3-Ⅰ to LC3-Ⅱ increased in cells transduced with wt PTEN and the Y138L mutant, but decreased in cells transduced with PTEN shRNA compared to control cells. These results indicate that the lipid phosphatase activity of PTEN enhances autophagy, which further stimulates the lipophagy triggered by DENV infection, resulting in the depletion of LDs. The mechanisms by which PTEN regulates autop-hagy and reduces LDs during DENV infection still requires clarification.
Taken together, these data show that the lipid phosphatase activity of PTEN regulates lipid metabolism through Akt/FoxO1/Maf1 signaling and autophagy to enhance DENV replication.
DENV Reduces Endogenous PTEN Expression through Post-Transcriptional Regulation
PTEN Overexpression Enhances DENV Replication
PTEN Lipid Phosphatase Activity Benefits DENV Production
The Lipid Phosphatase Activity of PTEN Controls LDs
PTEN/Akt/FoxO1/Maf1 Signaling Regulates Lipid Metabolism during DENV Infection
-
Previous studies have demonstrated that DENV infection triggers autophagy and lipophagy to deplete LDs, releasing FFAs for cellular β-oxidation and energy production (Heaton and Randall 2010). Up-regulated ancient ubiquitous protein 1 (AUP1) and AMP kinase/mTOR axis are required for the lipophagy induced by DENV (Zhang et al. 2018) but the precise mechanisms by which DENV induces lipophagy and regulates lipid metabolism remain elusive.This study showed that PTEN, specifically its lipid phosphatase activity, facilitates DENV infection by regulating lipid metabolism through the Akt/FoxO1/Maf1 signaling and autophagy induction, in Huh7 cells.
DENV infection leads to downregulated PTEN expression in infected Huh7 cells, and PTEN overexpression facilitates viral production but PTEN silencing acts the opposite function. Given the results from our study, one possible explanation is that PTEN down-regulation is seemingly a self-protective mechanism of target cells upon DENV infection. It is also supported by the similar results from our previous study showing that the downregulation of PTEN in DENV-infected human umbilical vein endothelial cells (HUVECs) reduced the expression of viral capsid protein and the release of progeny virus particles (Yu et al. 2015). As a tumor suppressor, PTEN regulates cell growth and survival through PI3K inhibition. The loss of PTEN in epithelial cells decreases the protective effects of interferon (IFN) against vesicular stomatitis virus (VSV), indicating crosstalk between the two signaling pathways (Yu et al. 2015). Moreover, HCV core of genotype 3a decreases PTEN expression, promoting LDs accumulation (Peyrou et al. 2013). The reduction of PTEN was observed in Japanese encephalitis virus (JEV) infected microglial cells by has-miR-374b-5p to regulate PI3K/Akt/IRF3 signaling (Rastogi and Singh 2019). The modulation tendency of PTEN by these viruses appear similar with DENV. However, our findings highlight differences in the molecular mechanisms by which viral infections regulate PTEN.
The intracellular membrane machinery is hijacked by positive-sense RNA viruses to aid replication (Tang et al. 2014). As dynamic organelles for lipids reservoirs, LDs are used by DENV to facilitate viral replication providing energy and a platform for viral assembly and encapsidation (Heaton and Randall 2010; Rodenhuis-Zybert et al. 2010; Perera et al. 2012; Mustafa et al. 2015; Randall 2018). Previous studies indicate that DENV increases the number of intracellular LDs and their diameter, which in turn sequesters DENV capsid to act as a scaffold for genome encapsidation (Samsa et al. 2009). Specific hydrophobic amino acids in the DENV capsid protein and perilipin 3 (PLIN3) at the LDs surface are responsible for the accumulation of DENV capsid onto LDs and their interactions (Martins et al. 2012). The interactions depend on a high concentration of potassium ions and the non-canonical function of the host GBF1-Arf-COPI system (Carvalho et al. 2012; Iglesias et al. 2015). A decreased LDs area is observed due to the release of FFAs for cellular β-oxida-tion in DENV infected cells, indicating that DENV uses the energy stored in LDs and triggers lipophagy to maintain viral replication. This proposal is further supported by the finding that DENV NS4A/4B interacts with unmodified AUP1 localized at the LDs and ER, and activates its acyltransferase domain to trigger lipophagy (Carvalho et al. 2012). According to our data, the LDs area and number decreased upon DENV infection in Huh7 cells, which was consistent with the proposed LDs depletion during DENV infection. PTEN, specifically its lipid phosphatase activity, exacerbated the reduction in LDs area caused by DENV. In contrast, PTEN silencing by shRNA significantly rescued the DENV triggered reduction in LDs area, which was consistent with previous studies showing that PTEN depletion triggers the formation of large LDs during HCV infection (Peyrou et al. 2013). However, the increase in LDs by PTEN silencing neither promoted DENV RNA levels nor virus production, suggesting the presence of other critical restrictive factors in the context of DENV infection.
Functions of PTEN differ according to its lipid and protein phosphatase activity. As a lipid phosphatase, PTEN negatively regulates PI3K/Akt signaling (Fig. 6), the major signaling pathway involved in cell growth and cancer development. Extensive studies have confirmed the important role of PTEN in regulating lipid metabolism (Stiles et al. 2004; Ortega-Molina and Serrano 2013; Li et al. 2014). The loss of PTEN leads to Akt accumulation and the activation of numerous downstream substrates (Song et al. 2012). FoxO1 and mTORC1 are key but opposing downstream targets of Akt that regulate the expression of Maf1 and SREBPs involved in lipogenesis and lipophagy (Johnson and Stiles 2016). Akt phosphory-lates and inhibits FoxO1 by preventing its nuclear localization and target gene expression, resulting in the suppression of lipogenic gene expression (Zhang et al. 2006). By contrast, PTEN phosphorylates and activates mTORC1, enhancing lipogenesis (Porstmann et al. 2008). Maf1, activated by FoxO1 and inhibited by mTORC1, mechanistically occupies the FASN and ACC promoter and downregulates both of the lipogenic enzymes (Pluta et al. 2001; Upadhya et al. 2002; Palian et al. 2014). FoxO1 and mTORC1 also regulates SREBP expression and activity, which induces the expression of enzymes involved in lipid biosynthesis against Maf1 (Porstmann et al. 2008). From our results, both FASN and ACC were downregulated by PTEN and the Y138L mutant, but upregulated by PTEN silencing, suggesting that the lipid biosynthesis during DENV infection is regulated by FoxO1 and Maf1. Surprisingly, no significant differences in SREBP and mTORC1 expression were observed in cells transduced with wt PTEN or the Y138L mutant (data not shown). Given the importance of SREBP and mTORC1 in the regulation of lipid metabolism, their unique roles in DENV infection require further elucidation. Our results demonstrate that PTEN, specifically its lipid phosphatase activity, reduced Akt phosphorylation at T308 and S473, and upregulated FoxO1 and Maf1. Thus, the lipid phosphatase activity of PTEN regulated lipid metabolism through Akt/FoxO1/Maf1 signaling to benefit DENV production.
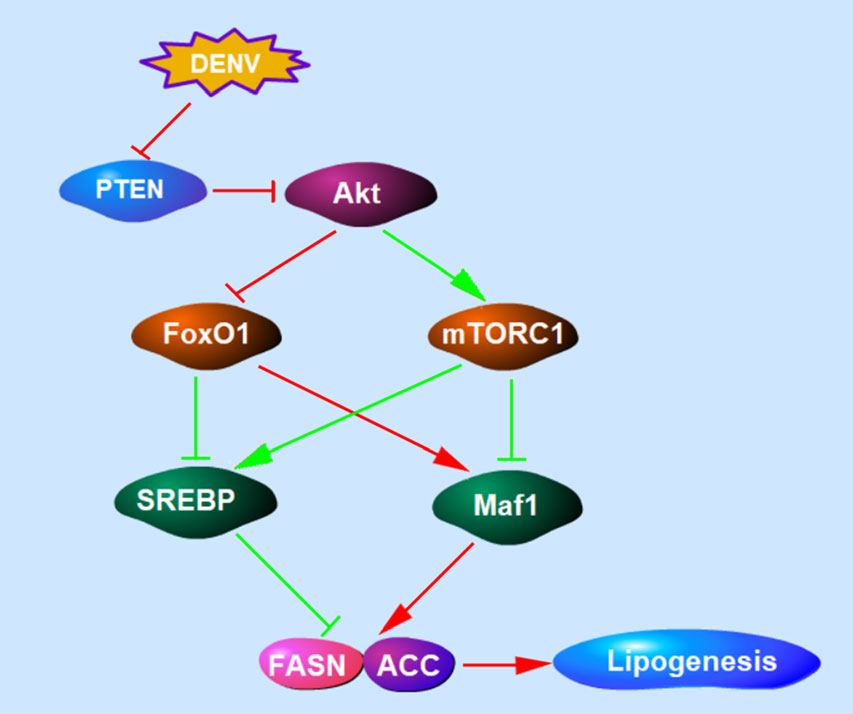
Figure 6. The schematic diagram of PTEN signaling pathway involved in lipogenesis. PTEN lipid phosphatase activity negatively regulates PI3K/Akt signaling. Akt inhibits FoxO1 and activates mTORC1. Maf1, activated by FoxO1 and inhibited by mTORC1, downregulates lipogenic enzymes including FASN and ACC. FoxO1 and mTORC1 can also regulate SREBP expression and induce the expression of lipogenic enzymes. The regulation of PTEN signaling pathway during DENV infection was showed in red lines.
Using focused RNA interference (RNAi) analysis, cellular pathways including autophagy and fatty acid biosynthesis were found to be required for DENV replication (Heaton et al. 2010). DENV co-opts fatty acid biosynthetic pathways to establish replication complexes. Rab18 coordinates the interactions of DENV-NS3 with FASN and the localization of LDs to facilitate DENV replication. It is speculated that a reduction of FASN in DENV infected cells expressing PTEN and the Y138L mutant correlates with the increased production of progeny virus due to enhanced consumption. On the other hand, PTEN and the Y138L mutant enhance autophagy induced by DENV, as indicated by the increased conversion of LC3-Ⅰ to LC3-Ⅱ. Autophagy-dependent processing of LDs releases FFAs for β-oxidation, resulting in their depletion. This was verified in this study, as autophagy induction mediated by PTEN and its Y138L mutant promoted LD depletion and enhanced virus production. The enhancement in autophagy regulated lipid metabolism through Akt/ FoxO1/Maf1 signaling through PTEN lipid phosphatase activity, which was coordinated to ensure a sufficient supply of lipids for DENV replication and assembly. Taken together, these findings provide mechanistic insight into the role of LDs during DENV infection.
-
This study was funded by the National Natural Science Foundation of China (81171564), the National Key Research and Development Program of China (2016YFC1200400) and the National S & T Major Project for Infectious Diseases Control (2017ZX10304403). The authors thank Yingfeng Lei (Fourth Military Medical University, China) for providing us polyclonal anti-DENV-2 antibodies for immunofluorescence assay.
-
QZT and QZL designed the experiments. LB, GTT, FXY, XZH carried out the experiments. QZL and LB analyzed the data and wrote the paper. All the authors approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.


















 DownLoad:
DownLoad: