HTML
-
Coronaviruses (Coronaviridae) are positive-sense single-stranded RNA viruses with enveloped virions (Masters and Perlman 2013). They can infect humans, other mammals, and birds, causing respiratory, enteric, hepatic, and neurological diseases of varying severity (Masters and Perlman 2013). Depending on the species, coronaviruses transmit via fomites or through aerogenic and/or fecal–oral routes (Liu et al. 2020). As coronavirus primarily target epithelial cells, they are generally associated with gastrointestinal and respiratory infections (Cong and Ren 2014). Coronaviruses are well known globally due to the emergence of severe acute respiratory syndrome (SARS) during 2002–2003 and Middle East respiratory syndrome (MERS) in 2012, both of which caused thousands of cases globally (Peiris et al. 2003; Bermingham et al. 2012).
Healthcare workers (HCWs) are at high risk of acquiring infection during occupational exposure to respiratory pathogens. For example, it has been reported that HCWs are at greatly increased risk of tuberculosis and seasonal influenza infection compared to the general population (Baussano et al. 2011; Kuster et al. 2011; Otter et al. 2016). The risk of HCW infection is significantly elevated during outbreaks of emerging infectious disease, in large part reflecting limited knowledge about the risk of transmission and the lack of appropriate protection. For example, during the 2002–2003 SARS outbreak, approximately 21% of infected cases were HCWs (Chowell et al. 2015), and this was as high as 62% and 51% in Hong Kong and Toronto (Lee et al. 2003; Booth et al. 2003). Infection of HCWs does not only result in a reduction in medical capacity, but it has also caused nosocomial transmission that generates high numbers of infections among other HCWs and patients. For example, during the MERS outbreak, some 24.1% of the cases were among HCWs, and 70.8% of these were associated with nosocomial infection (Chowell et al. 2015).
A novel pneumonia (COVID-19), caused by a novel coronavirus (SARS-CoV-2), was first reported in late December 2019 in Wuhan City, Hubei Province, China, and has since caused a global pandemic (WHO 2020a). Unfortunately, the ongoing pandemic of SARS-CoV-2 has already caused more than 3300 cases of HCW infection, 40% of which have occurred in hospitals in China (Information Office of the State Council of the People's Republic of China 2020). Although most COVID-19 disease among HCWs occurred during early stages of the outbreak, nosocomial infection is ongoing as the virus is spreading globally. For example, the nosocomial transmission of COVID-19 has recently occurred in Italy, resulting in about 70 cases of HCW infection since February 20, 2020 (Remuzzi and Remuzzi 2020). Accordingly, there is an urgent need to evaluate the risk of infection in hospitals and to take effective infection control procedures to protect HCWs against SARS-CoV-2.
Available data indicates that SARS-CoV-2 spreads more rapidly than SARS and MERS coronaviruses and has multiple transmission routes among humans (Chan et al. 2020; WHO 2020b; Kim et al. 2016). In particular, the virus largely transmits through respiratory droplets during the close contact with infected individuals (Chang et al. 2020). Infection also occurs when people contact with contaminated surfaces, and aerosol transmission remains a possibility. However, as many patients hospitalized with COVID-19 have been placed in airborne infectious isolation rooms (AIIR) with frequent air changes and routine hygiene processes, it is possible that hospitals have different modes and patterns of transmission than other environments. Recently, two studies reported the surveillance of environmental contamination during the hospitalization of SARS-CoV-2-infected patients, and suggested that surface contamination may be the main virus transmission route in AIIRs (Ong et al. 2020; Cheng et al. 2020). However, only preliminary results were reported due to small sample sizes and inconsistent methodology. In this paper we conducted a comprehensive environmental surveillance of AIIR at the Shanghai Public Health Clinical Center, evaluating the risk of infection in AIIRs during the treatment of SARS-CoV-2-infected patients.
-
To better understand the potential contamination of SARS-CoV-2 and the efficiency of hygiene procedure in airborne infectious isolation rooms (AIIRs), environmental samples were collected from floors, walls, washbasins, furniture, medical and personal protective equipment surfaces, as well as air samples, from AIIRs at the Shanghai Public Health Clinic Center (a COVID-19 designated hospital), Shanghai, China. The sampling sites covered all three regions in the inpatient area of the hospital: the clean area, the semi-contaminated area, and the contaminated area (Fig. 1; Supplementary Table S1).

Figure 1. Schematic diagram showing environmental sampling sites in the inpatient area of the Shanghai Public Health Clinic Center hospital. Arrows in blue show the direction of people's movement.
During February 8th to 24th, 2020, a total of 1544 environmental samples were collected from the inpatient area, with one to five replicates at each sampling site. For surface sampling, sterile swabs premoistened with viral transport medium (DMEM, without fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin) were used. Air sampling was performed using an automatic sampling system (Derenda PNS 16T-3.1) with a 46 mm membrane filter for 1.5 h at 1 m3/h for each sample in patient rooms, corridors, and changing rooms in both the building 1 and building 3 (Supplementary Table S2). In patient rooms, an air sampler was placed on the ground with a distance of about 1.0 m from patient's bed. In changing rooms, it was located between air supply outlet and air exhaust to capture particles from the unidirectional airflow. In addition, HEPA filters of air exhaust outlet in AIIRs in building 2 were collected.
Samples were collected before and after routine cleaning over a 4-week period. Furniture and medical equipment surface were sterilized twice daily using quaternary ammonium salt (0.22%–0.88%), while floor sterilization was performed once a day using 2000 mg/L of sodium hypochlorite. Personal protective equipment was sterilized repeatedly using disinfection gel with 54%–66% ethanol and 9%–11% n-propanol. All samples were stored at − 80 ℃ before RNA extraction.
-
Viral RNA of swab samples was extracted using a Magnetic beads nucleic acid isolation kit (Jiangsu Bioperfectus technologies Co. Ltd, Catalog no. JC10223-1) following the manufacturer's instructions. For air samples, the membrane filters were first cut into pieces and washed twice with 1.0 mmol/L NaOH (pH 10.8), followed by pH adjustment to 7.0–7.5 using a pH meter (Mettler Toledo, USA) and a concentration procedure using the Amicon Ultra-4 centrifugal filter with a membrane NMWL of 30 kDa (Milliore, USA). Viral RNA was then extracted from the concentrated eluent using a Viral RNA Mini Kit (Qiagen).
-
The presence of SARS-CoV-2 was detected by real-time RT-PCR targeting the RNA-dependent RNA polymerase (RdRp) gene as previously described (Wu et al. 2020). To determine the viral loads of clinical samples, RNA were extracted and measured in quantitative real-time RT-PCR using the Takara One Step PrimeScript RT-PCR kit (Takara RR064A) with forward primer 5′-GGGGAACTTCTCCTGCTAGAAT-3′, reverse primer 5′-CAGACATTTTGCTCTCAAGCTG-3′ and TaqMan probe 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3′ specifically targeting SARS-CoV-2 N gene. Serially-diluted plasmid pUC19 containing SARS-CoV-2 N gene (purchased from Sangon Biotech, Shanghai) were used as standard. RnaseP (RNP) house-keeping gene was measured with forward primer 5′-AGATTTGGACCTGCGAGCG-3′, reverse primer 5′-GAGCGGCTGTCTCCACAAGT-3′ and TaqMan probe 5′-ROX-TTCTGACCTGAAGGCTCTGCGCG-BHQ2-3′ as reference to normalize the total RNA amounts of individual samples. The SARS-CoV-2 viral loads in clinical samples were calculated with the Ct values extrapolated against the standard curve of plasmids. PCR reactions were performed using an ABI 7500 Real-Time PCR systems. Quantitative viral load of air samples and surface samples test with the BioDigital General dPCR kit (Jiangsu Saint Genomics, Cat no. CSJ-3-0018) following the product manual.
-
Differences in viral load between samples were estimated using a nonparametric Mann–Whitney test in SPSS (version 25.0). The R package ggplot2 was used to produce histograms. The sampling sketch map was produced using Adobe illustrator CC (version 22.0.0).
Study Design and Sample Collection
Sample Processing
Detection and Quantification of SARS-CoV-2
Statistical Analysis
-
Shanghai Public Health Clinical Center is the designated hospital to treat adult patients with COVID-19 in Shanghai. From January 20, 2020 to March 10, 2020, a total of 334 clinic- and laboratory-diagnosed patients have been hospitalized and treated in AIIRs with negative differential pressure in three independent buildings, denoted 1, 2 and 3. Relevant information on the AIIRs, patients and HCWs in each building is provided in Table 1. The 82 AIIRs in building 1 and 2 were non-ICU AIIRs dedicated to patients with mild symptoms. There were 58 HCWs (12 doctors and 46 nurses) in building 1 who were responsible for 111–113 patients, and 41 HCWs (10 doctors and 31 nurses) in building 2 responsible for 74–77 patients at the time of surveillance. The 33 AIIRs in building 3 were intensive care unit (ICU) AIIRs for patients in severe or critical condition. A total of 191 HCWs (30 doctors and 161 nurses) were responsible for 33 patients in building 3. All the surgery including intubation, tracheotomy and ECMO were performed in the AIIRs in building 3.
Parameter Building 1 Building 2 Building 3 AIIR information AIIR numbers 41 41 33 Beds in each AIIR 3 3 1 or 2 Air pressure (Pa) -20 -20 -20 Air change rate (per hour) 15 15 15 Operation daysa 17 11 24 Hospitalized patients Number of patients at first sampling 111 74 40 Number of patients at second sampling 113 77 32 Patient condition Mild Mild Severe, critical Surgery No No Intubation, tracheotomy, ECMO Healthcare workers Number of doctors 12 10 30 Number of nurses 46 31 161 AIIR airborne infectious isolation room, ECMO extracorporeal membrane oxygenation.
aFrom the date that the first patient was hospitalized to when the first sample was collected.Table 1. Information on airborne infectious isolation rooms (AIIRs) at the Shanghai Public Health Clinic Center.
A diagram of the floor layout in the inpatient area of the Shanghai Public Health Clinic Center is shown in Fig. 1. For each floor, the space was divided into a clean area (including offices, living and changing rooms for HCWs), a semi-contaminated area (including nurse station and connective corridors) and a contaminated area (including corridor, AIIRs and dedicated bathrooms). Access to the contaminated area was restricted along a unidirectional route. Supplies were unidirectionally transferred from the clean area to the contaminated area through pass-through chambers.
All the AIIRs have independent air supply and exhaust systems, with a ventilation of 15 air changes per hour. Air supply was located at the ceiling above beds and the exhaust was placed on the wall near the floor, creating a directional airflow from top-to-bottom in individual AIIRs (Fig. 1). Exhaust air was cleaned by passing through HEPA filters before emission. The AIIR maintained a negative air pressure of 20 Pa differential to the corridor to prevent potential pathogen leakage into the clean area. All the AIIRs had self-closing doors equipped with hands-free foot-operated openers.
-
Before access to the semi-contaminated area and contaminated area, all healthcare workers were required to wear personal protective equipment (PPE), including hooded disposable coveralls, N95 respiratory masks, goggles, face shield, double-layers gloves, and disposable boots (Fig. 1). They were also required to wear powered air-purifying respirators when performing surgery in the ICU-AIIR of building 3.
The hygiene procedure was performed as follows. The floor was mopped with 2000 mg/L sodium hypochlorite solution once per day and the surfaces of furniture, instruments and other objects were disinfected with 0.22%–0.88% quaternary ammonium salt solution twice per day. Personal hygiene care of the healthcare workers was performed by spraying 75% alcohol solution or disinfection gel composed of 65% alcohol and 11% propanol after they performed surgery or medical care services.
-
Viral transmission and the risk of infection may be altered due to the unique environment and hygiene procedure in AIIRs. Under natural conditions SARS-CoV-2 is largely transmitted through respiratory droplets (Chan et al. 2020). To evaluate the risk of airborne transmission inside AIIRs, we collected air samples from 15 AIIRs, including 7 ICU-AIIRs in building 3 and 8 non-ICU AIIRs in buildings 1 and 2. Two of the samples were collected in the ICU-AIIR of building 3 when tracheotomy surgery was performed. Importantly, none of the air samples tested positive for the presence of SARS-CoV-2 virus (Supplementary Table S2). We also collected surface samples from air exhaust and HEPEA filters and failed to detect any viral RNA. Similarly, air samples collected in the corridor or the changing rooms of the semi-contaminated area did not show any presence of virus (Supplementary Table S2). The absence of virus in air samples indicates that the risk of airborne transmission inside AIIRs is low.
Two studies have identified surface contamination with SARS-CoV-2 in isolated wards before environmental hygiene measures were imposed (Ong et al. 2020; Cheng et al. 2020). To evaluate the transmission risk of surface contamination, we collected a total of 1138 surface samples and tested the presence of viral RNA at different locations inside AIIRs, including floors, walls, furniture, bathrooms, medical equipment and personal equipment such as cell phones (Fig. 1; Table 2). Samples were collected twice before environmental hygiene procedures. Critically, we did not detect any viral RNA in most of the surface samples inside AIIRs, including floors, walls, furniture, medical equipment or patients' cell phones, indicating that environment hygiene was effective in eliminating the virus (Table 2). However, viral RNA was detected in samples collected from the foot-operated openers and the bathroom sinks (Table 2). The amounts of viral RNA were low but detectable (Fig. 2). Notably, these positive samples were found in both mild (1 AIIRs in building 1) and severe (3 AIIRs in building 3) patient rooms, although positive samples seemed to be more frequent in AIIRs with severe patients, especially those containing surgical patients. Patients living in the AIIR in building 1 had mild disease and could get around freely, while those living in the 3 AIIRS in building 3 exhibited severe symptoms and were undergoing mechanical ventilation.
Sampling sites Building 1 Building 2 Building 3 Total 1sta 2nd 1st 2nd 1st 2nd Patient's room 7/537 (1.30%) 1. Air outlet 0/5b 0/5 0/5 0/5 0/2 NA 0/22 2. Floor 0/11 0/5 0/16 0/5 0/10 0/2 0/49 3. Wall 0/5 0/5 0/7 0/5 0/5 0/3 0/30 4. Door handle 0/10 0/13 0/12 0/5 0/5 0/14 0/59 5. Foot operated opener 1/2 0/13 0/5 NA 0/3 3/14 4/37 6. Chair 0/5 0/5 0/6 0/5 0/4 0/3 0/28 7. Medical equipment 0/1 0/5 0/1 0/5 0/22 0/11 0/45 8. Personal equipment (mobile phone) 0/6 0/7 0/6 NA 0/1 NA 0/20 9. Bed edge 0/9 0/10 0/11 0/10 0/10 0/6 0/56 10. Bathroom floor 0/2 0/5 0/3 0/5 0/3 0/3 0/21 11. Shower set 0/4 0/5 0/6 0/5 0/5 0/3 0/28 12. Sink 1/5 0/9 0/7 0/5 0/5 2/13 3/44 13. Table including handle 0/5 0/5 0/8 0/5 0/6 0/3 0/32 14. Toilet surface 0/4 0/6 0/6 0/5 0/4 0/3 0/28 15. Pass-through box including handle 0/5 0/5 NA NA 0/2 NA 0/12 16. Air sample 0/4 0/1 0/9 NA 0/5 0/7 0/26 Corridor 13/310 (4.19%) 17. Floor 0/9 0/14 0/3 0/6 3/12 1/18 4/62 18. Wall 0/6 0/6 0/6 0/6 0/12 0/6 0/42 19. Door handle 2/6 NA 0/4 NA 1/12 NA 3/22 20. Foot operated opener 1/6 NA 0/6 0/6 3/6 NA 4/24 21. Medical equipment 1/6 0/17 0/16 0/9 0/13 0/18 1/79 22. Sink 0/6 0/15 0/4 0/6 0/12 0/18 0/61 23. Table including handle NA NA 0/8 NA 1/2 NA 1/10 24. Air sample 0/1 0/2 NA NA 0/1 0/4 0/10 Elevator 2/69 (2.90%) 25. Floor 0/8 0/3 0/2 0/3 0/6 0/3 0/25 26. Wall 0/1 0/2 0/1 0/3 0/1 0/1 0/9 27. Elevator button 0/4 1/10 0/3 0/3 0/4 1/11 2/35 Healthcare workers 3/258 (1.16%) 28. Face shield 0/10 0/5 0/6 0/3 0/10 0/8 0/42 29. Front of protective clothing 0/10 0/5 0/6 0/3 0/11 0/8 0/43 30. Back of protective clothing 0/1 NA 0/6 NA NA NA 0/7 31. Hand (with gloves) 0/11 1/13 0/6 0/3 0/10 0/20 1/63 32. Sleeves 0/9 0/5 0/6 0/3 0/10 0/8 0/41 33. Foot 0/10 0/13 0/6 1/3 1/10 0/20 2/62 a Different numbers refer to different sampling times. b It stands for the number of positive samples/the total number of samples. NA, not available Table 2. Detection of SARS-CoV-2 in environmental samples collected from the contaminated area of the hospital.
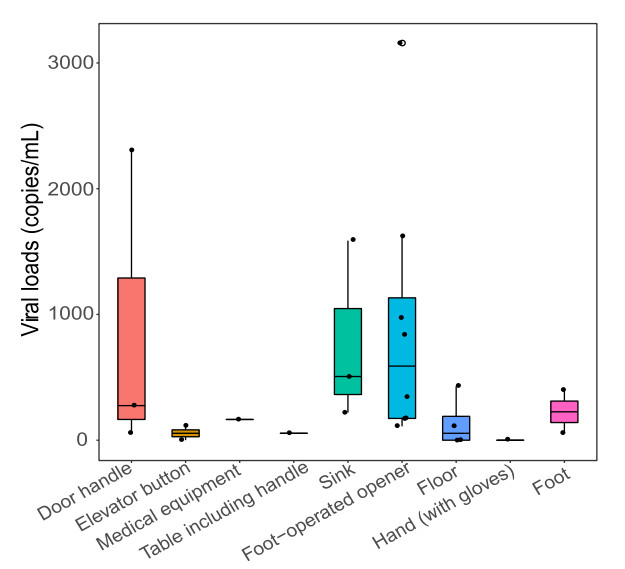
Figure 2. Viral loads in all the positive samples. The horizontal box lines represent the first quartile, the median, and the third quartile of viral loads. Each dot represents a positive sample and circles represent outliers.
The presence of viral RNA on foot-operated openers and the bathroom sinks may act as a source of contamination outside AIIRs. Accordingly, we collected and tested 61 samples in the corridors outside of AIIRs. As with testing inside AIIRs, viral RNA was also detected on the foot-operated openers in the corridors. Overall, 1 of 15 foot-operated opener samples in building 1, and 3 of 17 samples in building 3 were positive for SARS-CoV-2 RNA. Viral RNA was also detected in the samples collected on the corridor floor in building 3, but not in building 1 or 2. Furthermore, viral RNA was also detected on hand-touching objects, including the surfaces of door handles, medical equipment, furniture and elevator buttons in both buildings 1 and 3.
The presence of viral RNA on floors, foot-operator openers and hand-touching objects indicated that there was potential contamination of the gloves and boots of HCWs. We therefore tested the presence of viral RNA on the surface of the HCWs' PPE. Samples were collected from face shields or goggles, gloves, coverall sleeves, the front side of coveralls and bottom of disposable boots from 63 HCWs. Notably, samples were all collected just after HCWs provided medical care to patients and before disinfection (Fig. 1; Table 2). We did not detect any viral RNA on face shields, goggles, or on the front side of the HCW's coveralls. However, viral RNA was detected on the gloves of one nurse from building 1 (1/63) and two nurse's disposable boots in buildings 2 and 3 (2/62). The amounts of RNA were low, but detectable.
Although viral RNA was detected on the gloves and boots from some HCWs, all were required to remove PPEs and complete hand and boot hygiene before leaving the contaminated area. The chance of viral contamination of other areas was therefore very low. Indeed, no viral RNA was detected in 370 samples collected from semi-contaminated and clean areas, including floors, wall and other hand-touching surfaces (Supplementary Table S1).
-
According to the results of the environment surveillance, the environmental hygiene procedure was upgraded to more frequent floor-mopping, disinfecting the foot-operated openers and bathroom sinks, as well as more powerful personal hygiene of HCWs' gloves and boots with 75% alcohol with 0.10%–0.14% hydrogen peroxide solution. No viral RNA was detected in the samples collected following the application of the new infection control procedures.
At the time of writing, a total of 334 patients of COVID-19 have been hospitalized and treated at the center. Of these, 307 patients have been cured and discharged. All the off-duty HCWs received two throat swab tests for SARS-CoV-2 and they were isolated for medical observation for two weeks. None reported symptoms of COVID-19 infection or were positive for the presence of virus in throat swab tests.
Characteristics of AIIRs in Shanghai Public Health Clinical Center, Shanghai, China
Infection Control and Hygiene Procedures
Environmental Surveillance
Efficacy of Infection Control and Environment Monitoring
-
As the global pandemic of COVID-19 proceeds, there is an urgent need to develop effective infection control procedures to protect HCWs from SARS-CoV-2 infection. Herein, we describe the results of a comprehensive study of environmental surveillance from which we evaluated the risk of infection when working with confirmed COVID-19 patients in AIIRs at the Shanghai Public Health Clinical Center (Shanghai, China). These results will help infection control procedures in AIIRs for COVID-19 patients.
We found that the risk of viral transmission in AIIRs was different from that under natural conditions. Although SARS-CoV-2 primarily transmits through respiratory droplets (Chan et al. 2020; Liu et al. 2020), the chance of airborne transmission inside AIIRS was very low. The directional top-to-bottom airflow in AIIRs greatly reduced the transmission of respiratory droplets and the high air change rate prevented the accumulation of virus aerosols. Indeed, we did not detect any viral RNA from samples on the face shields or on front side of HCW's coveralls, or from the air samples collected inside the AIIRs. However, it is important to note that due to limitations in sampling and RNA detection, these results do not completely eliminate the possibility of airborne transmission of SARS-CoV-2 inside AIIRs. HCWs should clearly continue to wear N95 respiratory to protect themselves against viral infection.
Another important observation was that we observed the potential spread of SARS-CoV-2 through surface contamination occurred inside AIIRs. Viral RNA was detected on the surface of bathroom sinks, indicating possible contamination by patients' respiratory droplets. Sinks then became a new source of contamination, through the hand gloves of HCWs, to contaminate the surfaces of other objects when they perform routine cleaning and disinfection in the ward. More frequent environmental and hand hygiene will reduce viral transmission through contaminated surfaces.
Finally, we observed the presence of viral RNA on the foot-operated openers inside AIIRs and floors in corridors. Viral RNA was also detected in samples collected from HCWs' footwear. This is of particular note because footwear hygiene is often neglected in infection control procedures. In AIIRs, the directional top-to-bottom airflow may force respiratory droplets from infected patients to accumulate on the floor. Virus may then contaminate the footwear of HCWs and accumulate on the surface of foot-operated openers to become a new source of contamination. Therefore, the usage of disposable boots as footwear covers and more extensive footwear hygiene should reduce virus transmission through contaminated footwear. As viral culture was not performed, we were unable to assess the viability of viruses in collected samples. Additional work is therefore needed to determine whether these viral RNA positive samples also contained infectious material. However, this study clearly reveals that the surface of foot-operated openers is a potential new source of viral contamination in AIIRs. With the help of effective infection control procedures, virus transmission through contaminated footwear could be reduced. In addition, monitoring the environmental contamination of wards and disinfecting contaminated surfaces and places should be regularly performed to prevent the infection of HCWs.
-
We sincerely thank Doctor Cai-Hong Xu at Department of Environmental Science and Engineering of Fudan University for help in air sampling, and Ao-Jie Wang for experimental preparation. This study was supported by the Special National Project on investigation of basic resources of China (Grant 2019FY101500), the National Natural Science Foundation of China (Grants 81861138003 and 31930001). ECH is supported an Australian Research Council (ARC) Australian Laureate Fellowship (FL170100022).
-
YZZ, TYZ, and ZGS conceived and designed the study. ZGS, YMC, FW, LX, XC BFW, LS, and JLS collected samples. ZGS, YMC, FW, LX, FHD, and JMC conducted the experiments. BFW, and LS were responsible for hygiene procedure in airborne infectious isolation rooms. ZGS, YMC, FW, TYZ, and YZZ analyzed data. ZGS, FW, ECH, and YZZ wrote the manuscript with input from all authors. YZZ led the study.
-
All authors declare that they have no conflict of interest.
-
This study does not contain any studies with human participants or animals performed by any of the authors.

















 DownLoad:
DownLoad: