HTML
-
The emergence and re-emergence of RNA virus outbreak highlight the urgent need for the development of broadspectrum antivirals. Currently, broad-spectrum antivirals can be classified into several broad categories, including interferon and/or interferon inducers to stimulate host immune response, nucleotide analogs that resemble cellular DNA or RNA nucleotide and inhibit viral RNA replication, transcription or translation, and compounds or peptides that block entry of diverse viruses.
Polyamines, including putrescine, spermidine and spermine, are a class of positively charged small molecules derived from ornithine, and are ubiquitously and abundantly present in cells at concentrations up to 1.0 mmol/L (Igarashi and Kashiwagi 2000). Almost 80% of cellular polyamines directly bind to RNA (Igarashi and Kashiwagi 2015), and they have been found to participate in multiple cellular processes, including RNA folding, protein-RNA interactions, DNA structure formation, mRNA translation, and modulating membrane fluidity (Schipper et al. 2000; Gerner and Meyskens 2004; Mandal et al. 2013). Apart from various cellular processes, polyamines also play important roles in viral life cycles. It was reported that polyamines participate in the replication of a wide range of RNA viruses, such as enterovirus, flavivirus, alphavirus, vesiculovirus, bunyavirus, and filovirus (Mounce et al. 2016b; Kicmal et al. 2019; Mastrodomenico et al. 2019). Polyamines are also required for viral protein translation and keeping viral RNA-dependent RNA polymerase activity in the life cycle of viruses such as Chikungunya virus and Zika virus (ZIKV) (Mounce et al. 2016b, 2017). In addition, deletion of polyamine synthase suppressed the hypusination of eukaryotic initiation factor 5A that is required for both gene expression and viral replication of Ebola virus (Olsen et al. 2018). Moreover, polyamines were shown to be essential for maintaining the infectivity of Rift Valley fever virus and La Crosse virus, and polyamine depletion resulted in the accumulation of interfering noninfectious particles that limit infectivity (Mastrodomenico et al. 2019). Therefore, the biosynthetic process of polyamines has been considered as a potential ideal target for developing broad-spectrum antivirals against diverse RNA viruses, including many important human pathogens. For instance, difluoromethylornithine (DFMO), an FDA-approved specific inhibitor of ornithine decarboxylase (ODC1) that mediates the synthesis of polyamines from ornithine, has been found to inhibit the replication of various RNA viruses (Mounce et al. 2016b). However, targeting a pivotal host enzyme may result in an unexpected side effect, while directsequestration of polyamine may provide a rapid and reversible, alternative approach in restricting the replication of multiple RNA viruses.
Cucurbit[n]uril (CB[n], n = 5–8, 10, 14), a group of pumpkin-shaped macrocyclic glycoluril oligomers linked via methylene groups, possess a hydrophobic cavity fringed with two hydrophilic carbonyl portals that preferably bind with amine-based organic compounds with high affinity (Assaf and Nau 2015; Barrow et al. 2015). Within this family, CB[7] has attracted increasing attention for biomedical applications, attributed to its decent water solubility, biocompatibility and appropriate cavity size that can accommodate a variety of bioactive guest molecules, including amine-containing molecules such as polyamines (Shetty et al. 2015; Kuok et al. 2017). In particular, CB[7] has been shown to regulate the physiological functions of bioactive molecules through competitively inhibiting their binding with non-target and target biological receptors (Kuok et al. 2017; Zhang et al. 2019b; Yin et al. 2021). For instance, supramolecular encapsulation of polyamines by CB[7] was used to control polyamine-induced conformational changes of DNA, due to the higher binding between polyamines with CB[7] than that with DNA (Parente Carvalho et al. 2015; Wang et al. 2018; Chernikova and Berdnikova 2020). In addition, in vitro and in vivo sequestration of paraquat by CB[7] was recently shown to successfully reverse and control the toxicity of the guest species (Zhang et al. 2019a). Therefore, we hypothesized that CB[7] could potentially exert antiviral effects against RNA viruses via supramolecular encapsulation of endogenous cellular polyamines.
In this work, the broad-spectrum antiviral effects of CB[7] against diverse RNA viruses, including human enterovirus A71 (EV-A71), coxsackievirus A16 (CVA16), coxsackievirus B3 (CVB3), echovirus 11 (Echo-11), Semliki forest virus (SFV), dengue virus 2 (DENV2) and ZIKV were uncovered for the first time. The relationship between the antiviral activity of CB[7] and the polyamine pathway was also investigated.
-
Human rhabdomyosarcoma (RD) cells, human non-small cell lung cancer A549 cells, human colon adenocarcinoma Caco2 cells, human cervical carcinoma HeLa cells, human embryonic kidney HEK293T cells, green monkey kidney Vero cells and Baby Hamster Syrian Kidney BHK-21 cells were purchased from ATCC (American Type Culture Collection) and maintained in Dulbecco modified Eagle medium (DMEM; Gibco, China) supplemented with 10% fetal bovine serum (FBS; Gibco, Australia), 100 U/mL penicillin, and 100 g/mL streptomycin (HyClone; Austria) at 37 ℃ in an incubator with 5% CO2. Aedes aegypti Aag2 cells (ATCC, CCL-125) were cultured at 27.5 ℃ in Schneider's insect medium (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco). Aedes albopictus C6/36 cells (ATCC CRL-1660) were cultured in RPMI 1640 (Thermo Fisher Scientific, China) medium containing 10% FBS at 27.5 ℃. CB[7] (C42H42N28O14, molecular weight: 1162.96 g/mol) was synthesized as described previously (Kim et al. 2000; Bardelang et al. 2011).
-
EV-A71 strain H VR-1432 were obtained from ATCC, and CV-A16 GD09/24 were kindly provided by Dr. ChengFeng Qin (Beijing, China), and CVB3 Wooddruff was kindly provided by Dr. Zhaohua Zhong (Harbin, China). The clinical strain of EV-A71 (XY833) was obtained from Hubei Province Center for Disease Control and Prevention (Hubei, China). The clinical strain of Echo-11 (GWCMC01/GZ/CHN/2019) was obtained from Guangzhou Women and Children's Medical Center (Guangzhou, China). These viruses were amplified and titers were determined in RD cells. ZIKV strain GZ01 and DENV2 strain TSV01 were kindly provided by Dr. Cheng-Feng Qin and amplified in C6/36 cells and titrated by plaque assay in BHK-21 cells, respectively. SFV was generated from an infectious cDNA clone of SFV4 kindly provided by Dr. Tero Ahola (Helsinki, Finland) and amplified in 293T cells and titrated by plaque assay in Vero cells.
-
In mammalian cells, at the day of infection, the medium was changed with 2% FBS–DMEM, and then the viruses were added to cells at an MOI of 0.1. Total RNAs were extracted at 24 or 48 h post-infection (hpi) and subjected to qRT-PCR analysis. For plaque assays, Vero cells in 12-well plates were infected with supernatants from the infected-RD cells treated with CB[7]. The cells were cultured at 37 ℃ for 2 h to allow the adsorption of all the viruses. The supernatant was then replaced with MEM containing 2% FBS and 1% penicillin–streptomycin with isopycnic 1% low-melting-point agarose (Sigma-Aldrich, Japan). After incubation at 37 ℃ for 72 h, cells were fixed with 10% formaldehyde and stained with 0.5% crystal violet at 4 ℃ for 2 h.
-
Cells were harvested in lysis buffer (50 mmol/L Tris–HCl [pH 7.4], 150 mmol/L NaCl, 1% NP-40, 0.25% deoxycholate, and a protease inhibitor cocktail [Roche]). The lysates were then subjected to 12% SDS-PAGE and Western blotting according to our standard procedures (Zhou et al. 2020). The antibodies used in this study are included anti-tubulin (Protein Tech Group, 1:3000), anti-VP1 (Abnova, 1:1000), anti-Zika virus Envelope protein (GeneTex GTX133314).
RNA Extraction and qRT-PCR Viral or cellular RNAs were extracted using the Purelink RNA mini kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Virus genomic RNA was quantified by one step PrimeScriptTM RT-qPCR Kit (Takara, Japan). IFN pathway-related gene transcripts were quantified using a one-step SYBR green RT-PCR Kit (Takara).
-
ODC1 siRNA and SAT1 siRNA duplexes were purchased from RIBOBIO, China. Silencer Negative Control siRNA (siNC) was used as negative control and does not target any endogenous transcript. The siRNA transfection was performed according to the manufacture's instructions.
The cytotoxicity of CB[7] was detected by using a Cell Counting Kit (CCK-8, Beyotime, China) following the manufacturer's instruction. Briefly, 100 μL cells (1 × 105 mL-1) were seeded into the wells of a 96-well microtiter plate and incubated at 37 ℃ for 12 h. Then CB[7] at graded concentrations was added. After incubation at 37 ℃ for 24 h, 10 μL of CCK-8 solution were added, followed by an additional incubation for 2 h. The absorbance was measured at 450 nm.
-
GraphPad Prism 8.0 was used for statistical analyses. All experiments were repeated at least three times. Statistical analysis was carried out by unpaired t test. P value < 0.05 was considered statistically significant.
Cell Culture and CB[7]
Viruses
Virus Infection and Plaque Assays
Western Blotting
siRNA Transfection and CCK-8 Assay
Quantification and Statistical Analysis
-
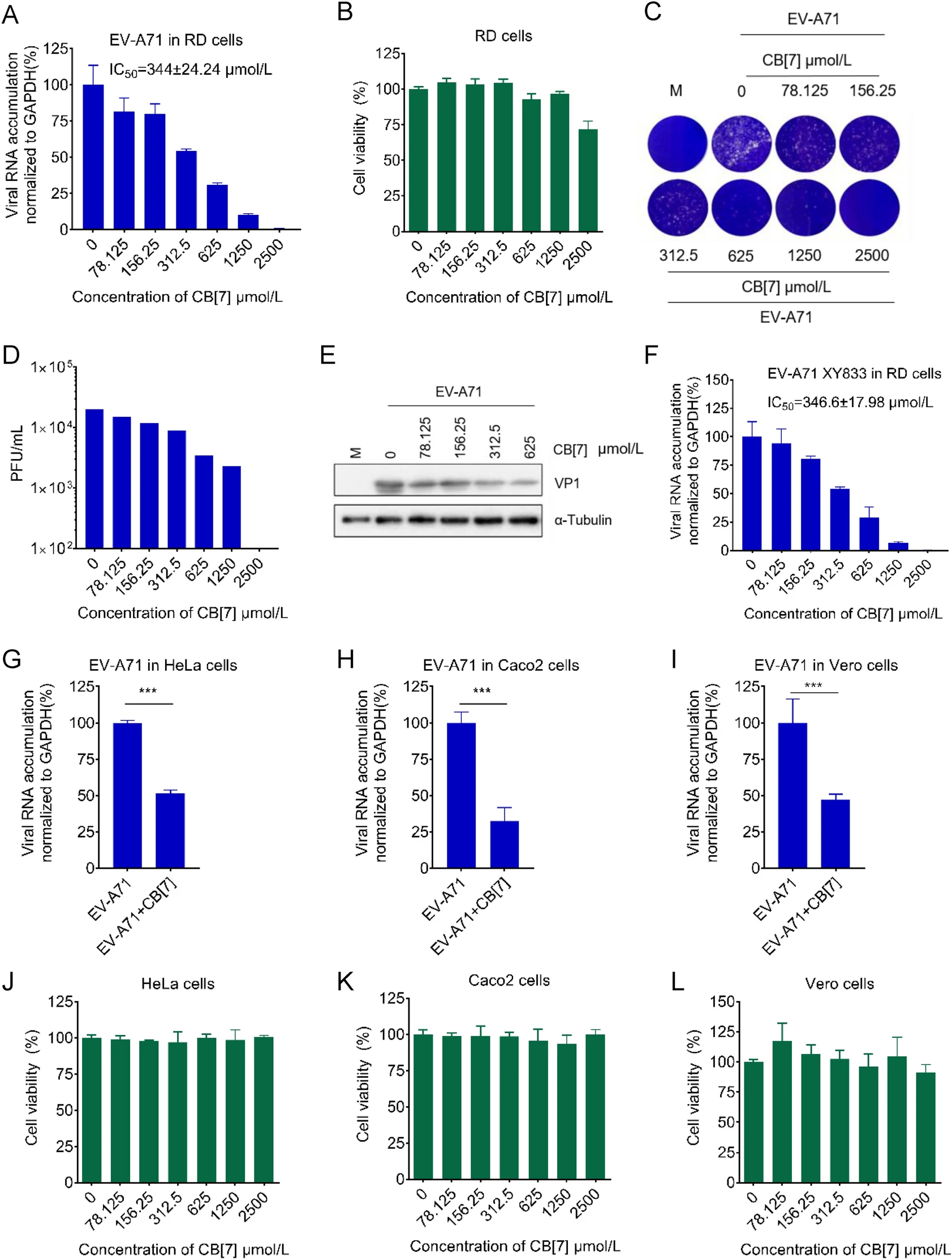
The supramolecular chemistry between CB[7] and polyamines have been well-studied and multiple evidence have shown that CB[7] exhibits higher binding affinity with different polyamines, including putrescine, spermidine and spermine, than nucleic acids (Table 1). Therefore, we sought to examine whether the sequestration of polyamines by CB[7] could exert any antiviral activity. To this end, EV-A71 that belongs to the genus Enterovirus of the family Picornaviridae and is the major causative pathogen for hand-foot-and-mouth disease (HFMD) was used for the initial investigation. We used EV-A71 (strain H, VR-1432) that is a common strain for laboratory research. Rhabdomyosarcoma (RD) cells were pretreated with increasing concentrations of CB[7] for 2 h and then infected with EVA71 for one hour at a multiplicity of infection (MOI) of 0.1. At 24 hpi, the antiviral effect of CB[7] was determined via measuring viral RNA accumulation with qRT-PCR. Our data showed that CB[7] exerted potent anti-EV-A71 activity in a dose-dependent manner, with the 50% inhibitory concentration (IC50) value of 344 ± 24.24 μmol/L (Fig. 1A). Besides, the cytotoxicity of CB[7] in RD cells was examined via CCK-8, and the 50% cytotoxic concentration (CC50) of CB[7] in RD cells is greater than 2500 μmol/L (Fig. 1B). Moreover, this anti-EV-A71 effect of CB[7] was also confirmed by measuring virus titers in infected cells via plaque assays (Fig. 1C, 1D) or determining EV-A71 coat protein VP1 via Western blotting (Fig. 1E), respectively. With the promising antiviral effects of CB[7] on the common strain of EV-A71 (strain H, VR- 1432), we further tested the effect of CB[7] on a clinical strain of EV-A71 (strain XY833) in RD cells, and found that the IC50 value of CB[7] against this clinical isolated strain is 346.6 ± 17.98 μmol/L. (Fig. 1F).
Polyamines Nucleic acids/CB[7] Methods Binding constants (Ka, M-1) References Putrescine CB[7] Fluorescence titration in 10 mmol/L NH4OAc buffer of pH 6.0 at ambient temperature 3.7 × 105 Hennig et al. (2007) Spermidine CB[7] ITC titration in 100 μmol/L CaCl2, NaCl and NaHCO3 at 25 ℃ 2.2 × 105 Da Silva et al. (2014) Spermine CB[7] ITC titration in 20 mmol/L PBS buffer of pH 6.0 at 37 ℃ 1.18 × 106 Chen et al. (2017) Putrescine DNA Affinity capillary electrophoresis method in 20 mmol/L Tris-HCl buffer of pH 7.0 at 25 ℃ 1.02 × 105 Ouameur and Tajmir-Riahi (2004) Spermidine DNA Affinity capillary electrophoresis method in 20 mmol/L Tris-HCl buffer of pH 7.0 at 25 ℃ 1.4 × 105 Ouameur and Tajmir-Riahi (2004) Spermine DNA Affinity capillary electrophoresis method in 20 mmol/L Tris-HCl buffer of pH 7.0 at 25 ℃ 2.3 × 105 Ouameur and Tajmir-Riahi (2004) Spermine DNA Gel filtration method in 100 mmol/L Tris-HCl, 2 mmol/ L Mg2+ and 100 mmol/L K+ buffer of pH 7.5 at 4 ℃ 103×105 Igarashi et al. (1982) Spermidine 16S rRNA Gel filtration method in 100 mmol/L Tris-HCl, 2 mmol/ L Mg2+ and 100 mmol/L K+ buffer of pH 7.5 at 4 ℃ 2.2 × 103 Igarashi et al. (1982) Spermine 16S rRNA Gel filtration method in 100 mmol/L Tris-HCl, 2 mmol/ L Mg2+ and 100 mmol/L K+ buffer of pH 7.5 at 4 ℃ 1.8 × 104 Igarashi et al. (1982) Table 1. Binding affinities of polyamines and CB[7] or nucleic acids.

Figure 1. CB[7] inhibits the replication of EV-A71. A RD cells were treated with CB[7] at various concentrations for 2 h, after that cells were washed by PBS and infected with EV-A71 at a MOI = 0.1 for 1 h, and then cells were washed by PBS for three times and CB[7] were added. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR. B Cell viability of RD cells determined with CCK-8 assay. C RD cells were pretreated with various concentrations of CB[7] before infection with EV-A71, and supernatant was collected at 24 hpi for plaque assay. D Quantification of viral titers. E Western blotting for EV-A71 VP1 protein in RD cells treated with CB[7]. F The antiviral effect of CB[7] against EV-A71 (XY-833) was determined as described in (A). G–I Antiviral effects of 625 μmol/L CB[7] for EV-A71 in HeLa cells, Caco-2 cells and Vero cells. J–L Cell viability of HeLa cells, Caco2 cells and Vero cells determined with CCK-8 assay. ***P < 0.001.
We further examined the inhibitory effects of CB[7] on EV-A71 in different cell lines, including human cervical carcinoma HeLa cells, green monkey kidney Vero cells, and human colon adenocarcinoma CaCo-2 cells, via measuring viral RNA accumulation. As shown in Fig. 1G–1I, CB[7] treatment at a concentration of 625 μmol/L for 2 h significantly inhibited EV-A71 replication in all these cell lines. Besides, the CC50 values of CB[7] in these cell lines were all higher than 2500 μmol/L (Fig. 1J–1L).
Next, we sought to examine the antiviral effects of CB[7] on other enteroviruses, including CV-A16, CVB3 and Echo 11 that are causative pathogens for HFMD, myocarditis and neonatal sepsis-like disease, respectively. We pretreated RD cells with CB[7] prior to infection with CV-A16, CVB3 or Echo 11, respectively, and viral RNA accumulation was determined via qRT-PCR. Our data showed that CB[7] treatment effectively inhibited the replication of CV-A16 (Fig. 2A) with the IC50 values of 433.3 ± 36.04 μmol/L and Echo 11 with the IC50 values of 276.1 ± 33.12 μmol/L (Fig. 2B), respectively. And CVB3 was also found to be sensitive to CB[7] (Fig. 2C). Together, our findings indicate that CB[7] exhibits a potent broad-spectrum antiviral activity against diverse enteroviruses.

Figure 2. CB[7] inhibits the replication of enteroviruses. A–B The antiviral effect of CB[7] against CV-A16 A in RD cells and Echo-11 B in HeLa cells were determined as described in Fig. 1A. C Antiviral effect of 625 μmol/L CB[7] for CVB3 in HeLa cells was measured via qRT-PCR. *P < 0.05.
-
To explore whether other RNA viruses are also sensitive to CB[7], we examined the antiviral activity of CB[7] against ZIKV that belongs to mosquito-borne flavivirus and is an important human pathogen for neonatal microcephaly (Yuan et al. 2017; Hu et al. 2019; Qiu et al. 2020). Human non-small cell lung cancer A549 cells were pretreated with various concentrations of CB[7] for 2 h before infection with ZIKV for 1 h at an MOI of 0.1, and the viral RNA accumulation was measuring via qRT-PCR at 24 hpi. We identified that CB[7] inhibited ZIKV replication in a dosedependent manner, with IC50 value of 285.6 ± 19.42 μmol/L in A549 cells (Fig. 3A). The CC50 value of CB[7] in A549 cells is more than 2500 μmol/L (Fig. 3B). Besides, this anti-ZIKV effect of CB[7] was also confirmed by determining ZIKV envelope protein via Western blotting (Fig. 3C) and plaque assays (Fig. 3D-3E). Consistently, CB[7] also showed anti-ZIKV effect in baby hamster kidney BHK-21 cells (Fig. 3F).

Figure 3. CB[7] inhibits the replication of Zika virus. A A549 cells were treated with CB[7] at various concentrations for 2 h, after that cells were washed by PBS and infected with ZIKV at a MOI = 0.1 for 1 h, and then cells were washed by PBS for three times and CB[7] were added. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR. B CCK-8 assay for the cell viability of A549 cells determined with CCK-8 assay. C Western blotting for Zika virus Envelope protein in A549 cells treated with CB[7]. D A549 cells were pretreated with various concentrations of CB[7] before infection with ZIKV, and supernatant was collected at 24 hpi for plaque assay. E Quantification of viral titers. F Antiviral effect of 625 μmol/L CB[7] for ZIKV in BHK cells was measured via qRT-PCR. ***P < 0.001.
Mosquito-borne flaviviruses were transmitted through vector mosquito to host mammals (Qiu et al. 2020). Thus, we sought to examine whether CB[7] could also inhibit ZIKV in mosquito cell lines, including A. aegypti Aag2 cells and A. albopictus C6/36 cells. As shown in Fig. 4A, 4B, CB[7] treatment efficiently inhibited ZIKV replication in these two cell lines, with the IC50 values of 426.5 ± 98.6 μmol/L in Aag2 cells and 560.1 ± 80.7 μmol/L in C6/36 cells, respectively. Besides, the CC50 values of CB[7] in these two cell lines are both greater than 2500 μmol/L (Fig. 4C, 4D).
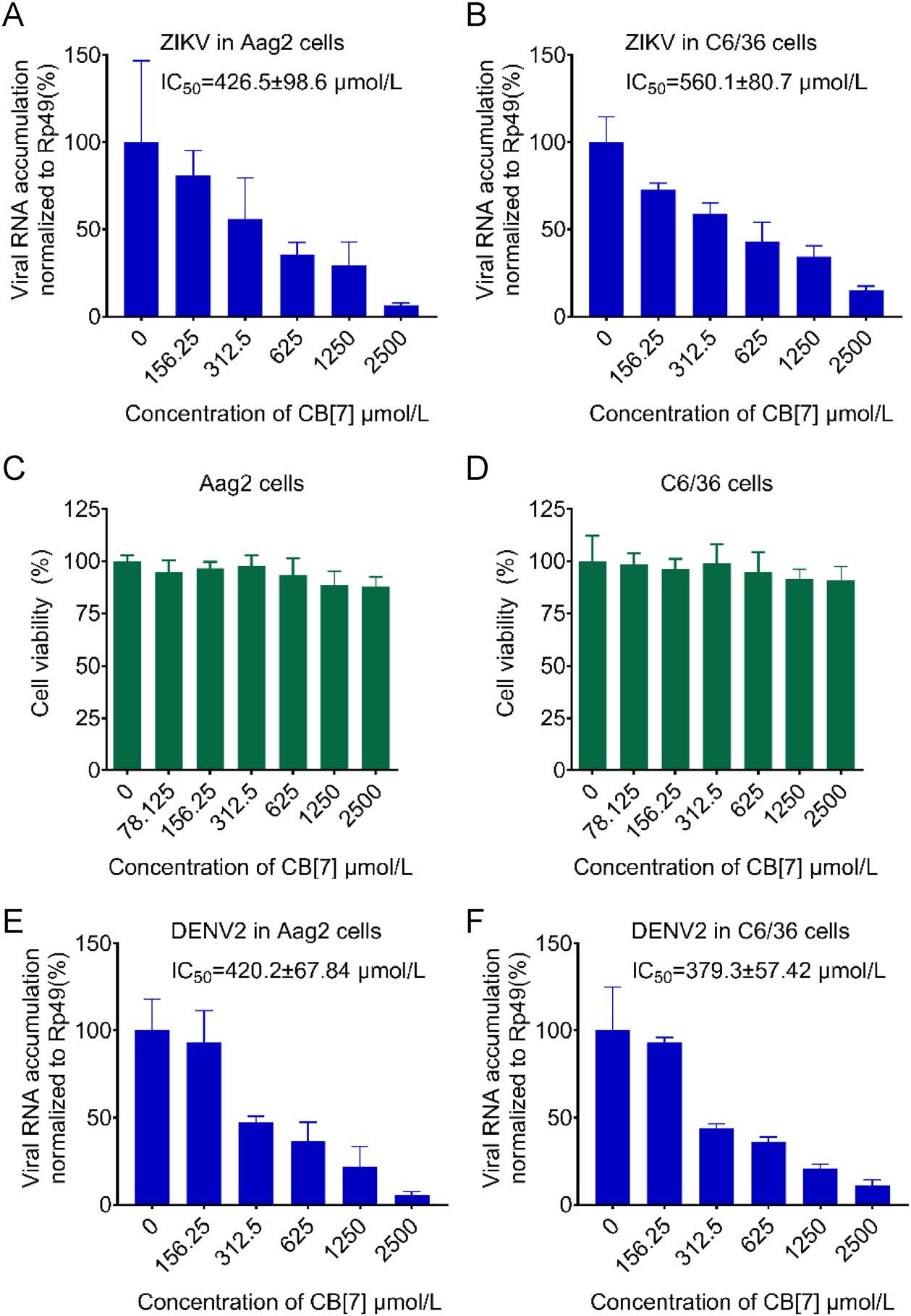
Figure 4. CB[7] inhibits the replication of flaviviruses in mosquito cell lines. A-B Aag2 (A) and C6/36 (B) cells were treated with CB[7] at various concentrations for 2 h, after that cells were washed by PBS and infected with ZIKV at a MOI = 0.1 for 1 h, and then cells were washed by PBS for three times and CB[7] were added. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR. C-D Cell viability of Aag2 cells and C6/36 cells determined with CCK-8 assay. Aag2 E and C6/ 36 F cells were treated with CB[7] at various concentrations for 2 h, after that cells were washed by PBS and infected with DENV2 at a MOI = 0.1 for 1 h, and then cells were washed by PBS for three times and CB[7] were added. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR.
We further examined the antiviral activity of CB[7] against DENV2, another important mosquito-borne flavivirus responsible for dengue hemorrhagic fever (Miao et al. 2019; Wang et al. 2020; Du et al. 2021), in Aag2 and C6/36 cells, respectively. As a result, the IC50 values of CB[7] anti-DENV2 are 420.2 ± 67.84 μmol/L in Aag2 and 379.3 ± 57.42 μmol/L in C6/36 cells, respectively (Fig. 4E, 4F). Together, our findings indicate that CB[7] can inhibit mosquito-borne flaviviruses in both mammalian and mosquito cells.
-
We sought to expand our findings in other RNA viruses. Thus, we examined the effect of CB[7] on SFV, a prototypic alphavirus, in human embryonic kidney 293T cells. Consistent with the antiviral effects of CB[7] on enteroviruses and flaviviruses, CB[7] exerts anti-SFV activity in 293T cells in a dose-dependent manner, with IC50 value of 603.4 ± 64.65 μmol/L and CC50 value of more than 2500 μmol/L (Fig. 5A and 5B). Collectively, our data demonstrate that CB[7] possesses the broad-spectrum antiviral effects against a wide range of RNA viruses in different cell lines.
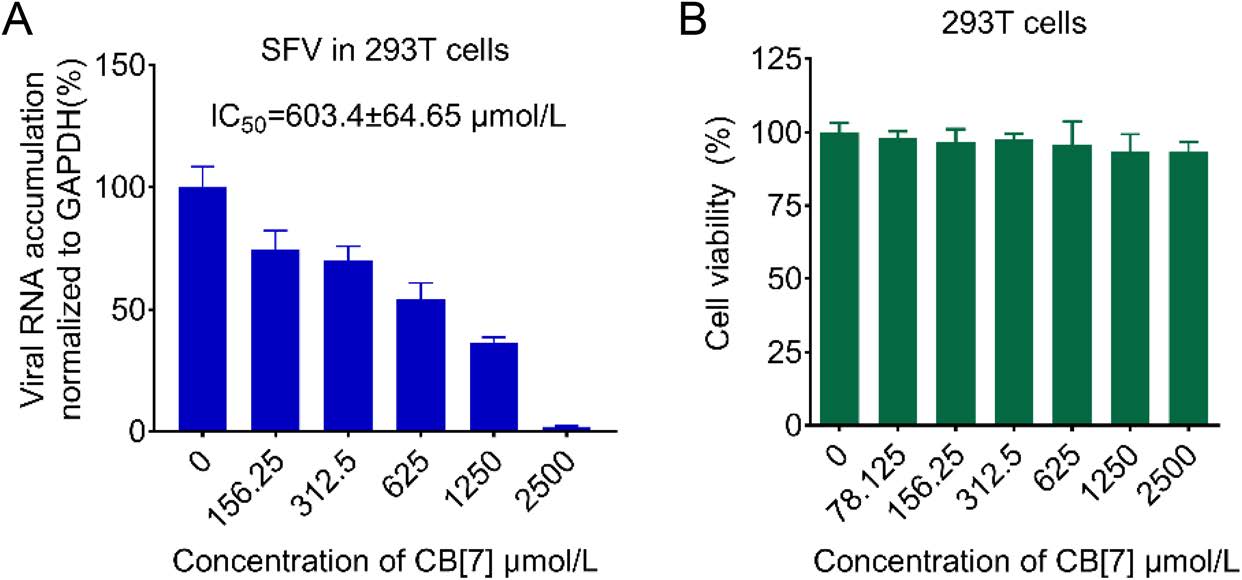
Figure 5. CB[7] inhibits the replication of alphavirus. A 293 T cells were treated with CB[7] at various concentrations for 2 h, after that cells were washed by PBS and infected with SFV at a MOI = 0.1 for 1 h, and then cells were washed by PBS for three times and CB[7] were added. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR. B Cell viability of 293 T cells determined with CCK-8 assay.
-
After identifying that CB[7] can inhibit viral RNA replication of diverse RNA viruses, we sought to examine whether the broad-spectrum antiviral activity of CB[7] is directly related to polyamine depletion. To this end, we adopted an RNAi-based loss-of-function to target ODC1, which mediates polyamines production (Pegg 2008; Mounce et al. 2016b). Interestingly, in the presence of ODC1 knockdown (siODC1; Fig. 6A), the treatment of CB[7] failed to further inhibit the viral RNA accumulation of EV-A71 in RD cells (Fig. 6B), indicating that the antiEV-A71 effect of CB[7] was absent in ODC1-deficient cells. Similarly, our data also showed that knockdown of ODC1 in A549 cells (Fig. 6C) impaired the antiviral activities of CB[7] against DENV2 and ZIKV (Fig. 6D–6E). Therefore, the antiviral activity of CB[7] is dependent on the polyamine pathway.
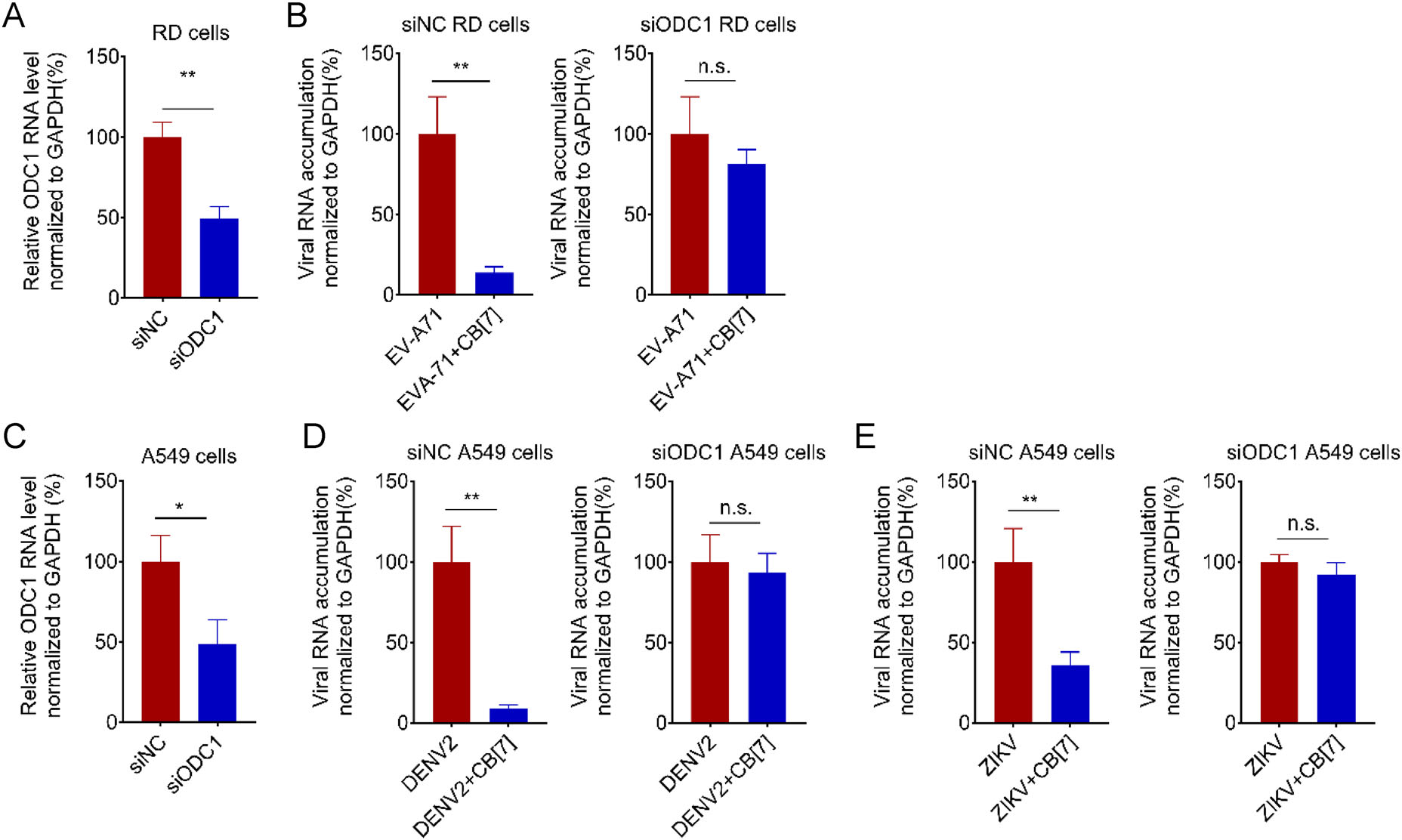
Figure 6. The antiviral activity of CB[7] relies on the polyamine pathway. A ODC1 mRNA level in RD cells after transfected with 50 μmol/L of siODC1 for 24 h. B 50 μmol/L siRNAs targeting cellular ODC1 (siODC1) and 50 μmol/L negative control siRNAs (siNC) were transfected into RD cells, respectively, for 24 h. After that, the siRNA-transfected cells were pretreated with 1250 μmol/L CB[7] for 2 h and following infection with EV-A71 at an MOI of 0.1. C ODC1 mRNA level in A549 cells after transfected with 50 μmol/L of siODC1 for 24 h. D–E 50 μmol/L siRNAs targeting cellular ODC1 (siODC1) and 50 μmol/L negative control siRNAs (siNC) were transfected into A549 cells, respectively, for 24 h. After that, the siRNA-transfected cells were pretreated with 1250 μmol/L CB[7] for 2 h and following infection with DENV2 (D) or ZIKV(E) at an MOI of 0.1, respectively. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR with t-test (GraphPad Prism). *P < 0.05; **P < 0.01; n.s., no significance.
-
Moreover, we tested the effects of CB[7] on diverse RNA viruses in the presence of DFMO, an FDA-approved specific enzymatic inhibitor of ODC1. As shown in Fig. 7A–7D, DFMO treatment significantly inhibited the viral RNA replication of DENV2, ZIKV, CVB3 and Echo 11 in A549 and HeLa cells, respectively, in line with the previous observations (Mounce et al. 2016b; Kicmal et al. 2019). Moreover, our data showed that the antiviral effects of CB[7] against these viruses were remarkably inhibited in cells pre-treated with DFMO (Fig. 7E–7H).
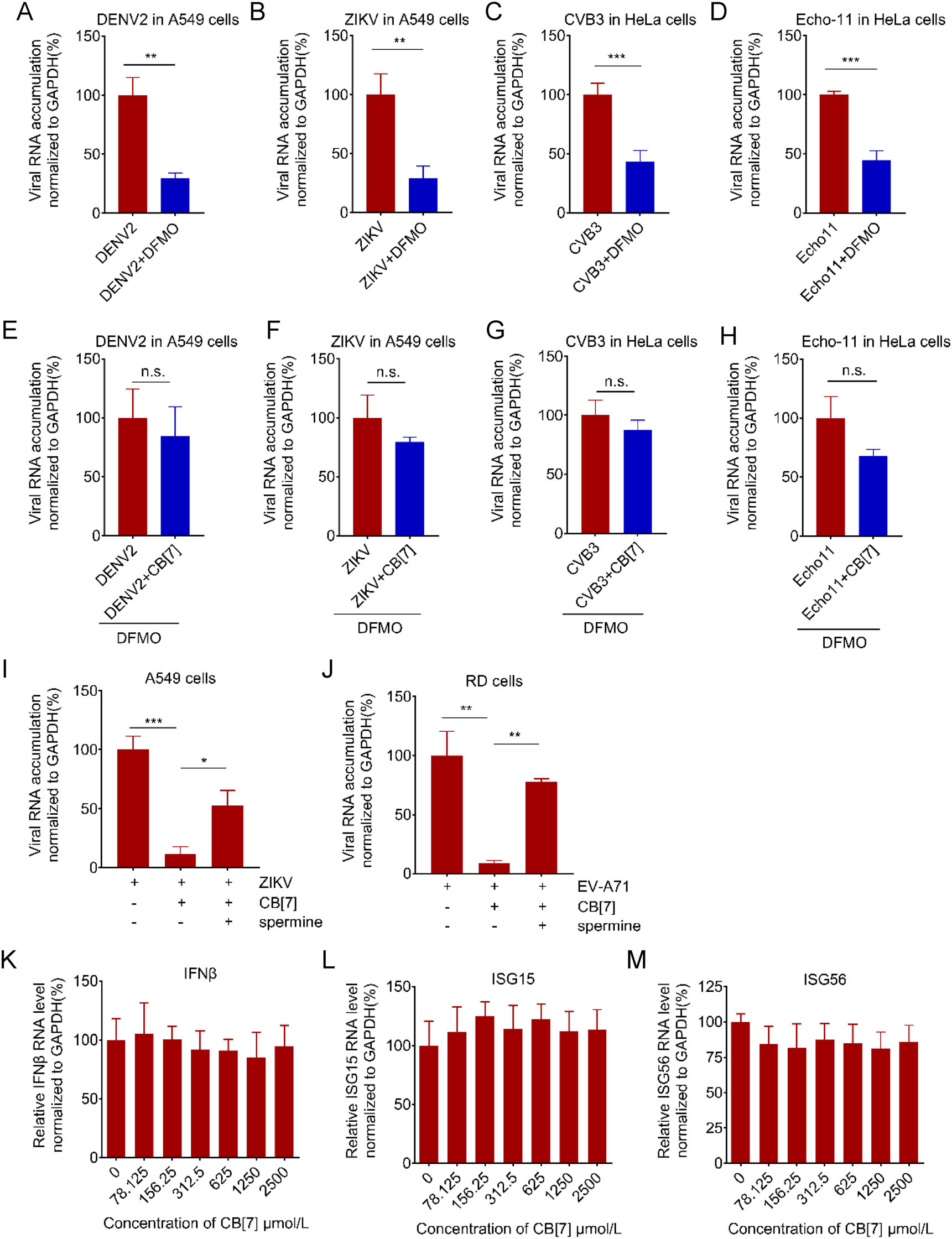
Figure 7. Supplying additional polyamine impairs the antiviral activity of CB[7] and CB[7] shows no effect on the IFN system A–D 500 μmol/L DFMO were added to the indicated cells for 72 h, after that cells were infected with DENV2 (A), ZIKV (B), CVB3 (C) and Echo 11 (D) for 1 h, respectively. Cells were then washed with PBS and DFMO were added back to cells for additional 24 h. Total RNAs were extracted at 24 hpi and subjected to qRT-PCR. E–H The indicated cells were pretreated with 500 μmol/L DFMO for 72 h and then treated with 625 μmol/L CB[7] for 2 h, following infection with DENV2 (E), ZIKV (F), CVB3 (G) and Echo 11 (H). Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR with t-test (GraphPad Prism). I–J A549 (I), RD (J) were treated with 1250 μmol/L CB[7] or 1250 μmol/L CB[7] plus 500 μmol/L spermine for 2 h before cells were infected with ZIKV or EV-A71. Total RNAs were extracted at 24 hpi and viral RNA accumulation was measured via qRT-PCR with t-test (GraphPad Prism). K–M A549 cells were treated with different concentrations of CB[7] for 24 h, total RNAs were extracted to test the mRNA level of indicated genes. **P < 0.01; ***P < 0.001; n.s., no significance.
To further confirm that the antiviral activity of CB[7] relies on polyamine, we sought to test the possibility that supplying additional polyamine could restore viral replication inhibited by CB[7]. Our data showed that supplementation of spermine restored the restricted replication of ZIKV and EV-A71 in A549 and RD cells treated with CB[7], respectively (Fig. 7I–7J). In addition, CB[7] treatment showed no effect on the gene expression levels of IFN-β as well as ISGs (Fig. 7K–7M), indicating that the antiviral activity of CB[7] is not due to innate immune induction.
In summary, our findings demonstrate that CB[7] has a broad-spectrum antiviral activity against diverse RNA viruses through sequestrating polyamines.
CB[7] Inhibits the Replication of Enteroviruses
CB[7] Inhibits the Replication of Flaviviruses
CB[7] Inhibits the Replication Alphavirus
Inhibition of the Polyamine Pathway Restricts the Antiviral Activity of CB[7]
CB[7] Inhibits the Replication of RNA Virus through Sequestrating Polyamine
-
The increase in emergence and re-emergence of RNA virus outbreaks has underlined the urgent need to develop effective broad-spectrum antivirals for diverse RNA viruses (Metsky et al. 2017; Henry and Vikse 2020; Tuite et al. 2020). In this study, we provide the first demonstration that CB[7], a novel macrocyclic molecule, exhibits a potent broad-spectrum antiviral activity against a wide range of RNA viruses, including many important human pathogens. Mechanistically, we identified that the antiviral effect of CB[7] is dependent on polyamines.
CB[n] is a novel molecular container that could form stable complexes with various molecules, including drugs, amino acids, polyamines, peptides, and even high-molecular-weight molecules like proteins (Assaf and Nau 2015). Moreover, the strong host–guest binding between CB[n] and spermine was well demonstrated in supramolecular chemotherapy to realize the controlled release of the included drugs towards spermine overexpressed cancer cells (Chen et al. 2017). We noticed that the binding constant of polyamine with RNA is much low than that with CB[7] (Chernikova and Berdnikova 2020). Taking advantage of this feature, CB[7] has the potential to be a promising broad-spectrum antiviral drug that can restrict the replication of multiple RNA viruses through complexing and sequestering polyamines.
Previously, we have successfully demonstrated that CB[7] could inhibit/reverse the toxic effects of a series of exogenous bioactive molecules, including neurotoxins (Li et al. 2015; Zhang et al. 2019a), pesticides (Huang et al. 2018), anesthetics (Zhang et al. 2019b) and so on (Yin et al. 2021). Of note, oral administration of paraquat (PQ), a highly toxic pesticide, together with CB[7] in a mouse model significantly decreased PQ levels in the plasma and major organs and alleviated major organs' injuries (Zhang et al. 2019b). Moreover, oral administration of CB[7] within 2 h post-ingestion of PQ also significantly increased the survival rates and extended the survival time (Zhang et al. 2019b), suggesting that CB[7] can be applied orally as an antidote. In addition, we demonstrated that CB[7] injected orally, peritoneally and intravenously administered with the dose as high as 5 g/kg, 150 mg/kg and 500 mg/kg, respectively (Zhang et al. 2018). These previous studies indicate that CB[7] exhibits excellent safety profiles in mice, which provide important foundations for further investigations and clinical applications of CB[7]. Our future work will test the potential of CB[7] as antivirals in vivo.
The polyamine pathway plays pleiotropic roles at the different stages during viral infections for a wide range of RNA viruses. Accumulating evidence indicates that polyamines' biosynthetic process can be ideal targets for developing broad-spectrum antivirals against diverse RNA viruses, including many important human pathogens. Previous studies showed that DFMO treatment can efficiently suppress a wide range of RNA viruses by inhibiting polyamine synthesis enzyme ODC1 (Mounce et al. 2016a, 2016b). Herein, we provide an alternative approach to inhibit the infections of various RNA viruses by directly encapsulating polyamines with a synthetic receptor, CB[7], instead of inhibiting various polyamine synthase such as ODC1 that may influence other cellular functions (Gibson and Roizman 1971; Schipper et al. 2000; Gerner and Meyskens 2004; Choi et al. 2016; Jiang et al. 2018; Routhu et al. 2018; Sandusky-Beltran et al. 2019). Besides, considering that the physical and chemical properties of the guest drugs are often improved as a result of complexation, CB[7] combined with antiviral drugs with different antiviral mechanisms may have synergistic antiviral effects.
In summary, this work demonstrates that macrocyclic CB[7] exhibits a broad-spectrum antiviral effect against a wide range of RNA viruses by sequestrating polyamines. Our findings not only broaden the potential biomedical application of CB[7], but also provide a novel broadspectrum antiviral drug. Via this action mechanism, other more specific artificial receptors of polyamines could be designed and synthesized as a new class of broad-spectrum antiviral drugs.
-
We thank Dr. Cheng-Feng Qin (Beijing, China), Dr. Zhaohua Zhong (Harbin, China), Dr. Kun Cai (Hubei, China), Dr. Yi Xu (Guangzhou, China), Dr. Tero Ahola (Helsinki, Finland) and Dr. Martub L. Moore (Georgi, USA) for kindly providing materials. This work was supported by the Strategic Priority Research Program of CAS (XDB29010300 to X.Z.), International Partnership Program of Chinese Academy of Sciences (153B42KYSB20200004 to X.Z. and R.W.), National Natural Science Foundation of China (21871301 to R.W., 81873964 to Y.Q. and 31970169 to X.Z.), Grant from the CAS Youth Innovation Promotion Association (2020332 to Y.Q.). We are also grateful to the Science and Technology Development Fund, Macau SAR (0007/2020/A to R.W.) and the Science and Technology Bureau of Wuhan (2018060401011309 to X.Z.) for providing partial support to this work.
-
JQ performed the experiments with the help of XJZ, YD and SL. YQ, RW and XZ designed the experiments, interpreted the results and wrote the manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.















 DownLoad:
DownLoad: