-
For the past few years specific regulatory T lym-phocytes including double negative T cells (DN Treg cells), CD4+CD25+ T cells and CD4+cytotoxic T lymphocytes have been the focus of immunological studies due to their roles in the development of many diseases. DN Treg cell, a recently identified regulatory T lymphocyte whose surface marker is TCRαβ+CD4-CD8-CD25+CD28-CD30+CD44-, shows specific cyto-toxic effects on syngeneic MHC-antigen complex CD8+ T cells through Fas/FasL interaction and extended graft survival time after activation (18). CD4+CD25+ T cells whose surface marker is TCRαβ+ CD3+CD4+ CD25+ CD45RO+ CTLA-4+could inhibit internal and external antigen induced immune responses after activation through the mechanism of inhibition cytokines and cell-cell contact (1, 5, 6, 10, 12), and thus plays an important role in the pathogenesis of infection, oncosis and autoimmune diseases (3, 7, 9).
Recent studies showed that in the process of viral infection, induced tissue damage and virus clearing, T lymphocyte plays an important role (3, 9). Virus hepatitis is a process of immunity induced hepatocyte inflammation damage. Until now, the understanding of Virus hepatitis immunopathogenesis remains at the level of CD8+ cytotoxic T lymphocytes (CTL). Further studies of the modulation factor of CD8+ CTL still are inconclusive. Qin et al constructed a C3H/Hej mouse chronic viral hepatitis model in which C3H/Hej mice individually received 10 PFU MHV-3 intra-peritoneally (8). In this study, we observed the changes of virus titer and pathology in livers of the C3H/Hej mouse chronic viral hepatitis model. Then we observed the ratios of T cell subsets of total T lymphocytes including the ratios of CD3+CD4-CD8-, CD3+CD4+CD8-CD3+CD4-CD8+, CD3+CD4+ CD25+T cell in the models.
HTML
-
4-6 weeks old C3H/Hej mice (weight 21±2g) were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Science and bred in the intellectual independent ventilating cleaning cage (IVC) in conditions of constant temperature and humidity with sterile food and acid water.
-
MHV-3 was obtained from Laboratory of Infectious Immunology, Tongji Hospital of Tongji Medical College, Huazhong University of Science & Tech-nology. Fluorescein isothiocyanate (FITC) labeled rat anti-mouse CD3-monoclonal antibodies, phycoery-thrin (PE) labeled rat anti-mouse CD4-monoclonal antibodies, Peridinin chlorophyll protein (Per CP) labeled rat anti-mouse CD8-monoclonal antibodies, Allophycocyanin (APC) labeled rat anti-mouse CD25-monoclonal antibodies were purchased from Santa Cruz Company.
-
XDS-1B microscope was purchased from the Chongqing Optical Instrument Factory. ELX-800 flow cytometer was purchased from Becton and Dickinson Company.
-
30 C3H/Hej mice which were used as normal control group were individually injected with 10 μL liquor natrii chloridi isotonicus and 90 C3H/Hej mice which represented the virus injection group were individually injected with 10 PFU MHV-3 intra-peritoneally. The blood, spleen and liver were collected at 0, 2, 4, 6, 8, 10, 12, 15, 20, 25, 30, 40 days.
-
We randomly selected a subset of livers from control and virus group. The livers were homogenated adequately by homogenizer and the supernatants were collected, another group of the livers were collected in 10% formalin and embedded with paraffine and sliced, then standard plaque assays and HE staining were performed.
-
100μL blood was obtained from a C3H/Hej mouse and collected in a plastic flow cytometry centrifuge tube, and 0.4μg of FITC-anti-CD3, PE-anti-CD4, PerCP-anti-CD8, APC-anti-CD25 monoclonal antibodies each were added. Another 100μL blood was collected to add homeotype comparison antibodies. The samples were incubated for 45 min at 4℃ with protection from light, and afterwards red blood cell lysis solution was added to lyse the red blood cells. The samples were then centrifuged for 10 min at 1500r/min. After removing the supernatant, cells were resuspended with 2mL 4℃ phosphate-buffered saline (PBS). Then the samples were washed twice with 2 mL 4℃ PBS, centrifuged for 10 min at 1 500r/min, resuspended by 500μL 1% paraformaldehyde-PBS and preserved at 4℃ with the protection from light. Flow cytometry was then performed.
-
The spleen of a C3H/Hej mouse was obtained in a sterile manner and ground on a 200 hole nylon membrane with flushed with Hanks buffer. After-wards, the samples were filtered and centrifuged for 5 minutes at 1 500r/min and the supernatant was then removed, red blood cell lysis solution was added to dissolve the red blood cell, and the samples were washed twice with 2 mL PBS at 4℃. Cell concen-tration was adjusted to about 2×107 /mL. 100μL single spleen cell suspension was collected in a plastic flow cytometry centrifuge tube. 0.4 μg of FITC-anti-CD3, PE-anti-CD4, PerCP-anti-CD8, APC-anti-CD25 mono-clonal antibodies were each were added into to the suspension. Another 100μL single spleen cell sus-pension was obtained to add homeotype comparison antibodies. The samples were incubated for 45 min at 4℃ and protected from light. Afterward, the samples were washed twice with 2 mL phosp-hate-buffered saline (PBS) at 4℃, then resuspended by 500μL of 1% paraformaldehyde-PBS and preserved at 4℃ with protection from light. Flow cytometry was then performed.
-
The livers of C3H/Hej mouse were obtained in a sterile manner and ground on a 200 hole nylon membrane with PBS. Afterward, the samples were filtered and centrifuged for 1 min at 540r/min. Then the supernatant was collected and centrifuged for 10 min at 1 700r/min. After removing the supernatant, the cells were resuspended with 2mL 40% Percoll buffer. After that, 2mL 70% Percoll was slowly dropped into the solution, and it was centrifuged for 20 min at 2 400r/min. Then the interlayer cells were collected and twice washed with 2mL PBS Cell concentration was adjusted to about 7.5×106/mL. Then the cells were labeled with fluorescent antibodies (same as above). Afterward, the samples were washed twice with 2 mL, PBS at 4℃, then resuspended by 500μL 1% paraformaldehydePBS and preserved at 4℃ away from light. Flow cytometry was then performed.
-
Virus titer in the liver tissue was tested by standard plaque assay at various time points. The liver samples were sliced and stained with HE and histological tests were performed using XDS-1B microscope.
Flow cytometric measurements of the ratios of CD3+CD4+CD25-, CD3+CD4-CD25+, CD3+CD4-CD25-, CD3+CD4+CD25+ T cell and CD3+CD4+CD8-, CD3+ CD4-CD8+, CD3+CD4-CD8-T cell were performed under standardized conditions using a ELX-800 flow cytometer equipped with a 488-nm argon laser. All measurements were performed in duplicate to assess intraassay variability.
-
(SPSS12.0) was used to analysis the data and sta-tistical significance was determined using the paired-samples T Test with P < 0.05. Intra-assay reproduci-bility was derived from the duplicate samples by one-way analysis of variance using Student-Newman-Keuls test.
Laboratory animals
Reagents
Instrument
Preparation of blood, spleen and liver
Preparation of liver samples for HE staining and standard plaque assay
Preparation of blood samples for flow cytometry
Preparation of spleen samples for flow cytometry
Preparation of liver samples for flow cytometry
Measurements
Statistical analysis
-
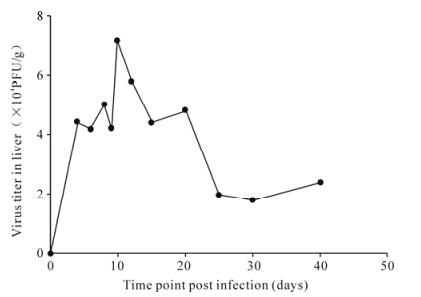
The virus titer in livers showed an significant increase 2 days post MHV-3 infection in C3H/Hej mice, while no virus titer was detected in the normal control group (Fig. 1).
The pathologic changes in livers were manifested as hepatocyte swelling, hydropic degeneration, spotty necrosis and infiltration of T cells 2 days post MHV-3 infection in C3H/Hej mice, while no pathologic change was detected in the normal control group (Fig. 2).
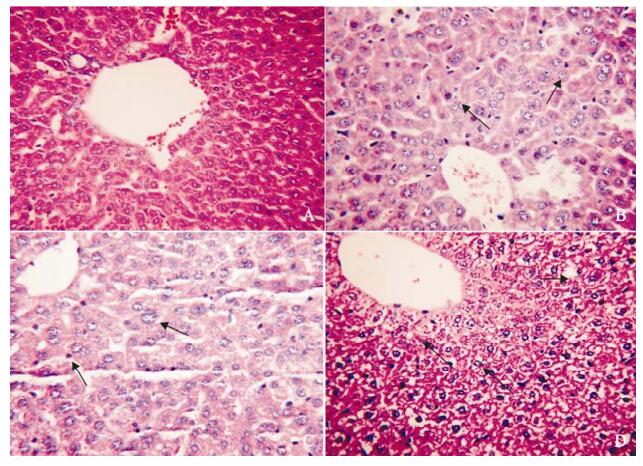
Figure 2. The pathological change in liver in MHV-3 infected C3H/HeJ mice. A: control; B: 4 days post MHV-3 infection; C: 12 days post MHV-3 infection; D: 40 days post MHV-3 infection. The arrows in the figures show hepatocyte swelling, hydropic degeneration, spotty necrosis and infiltration of T cells.
-
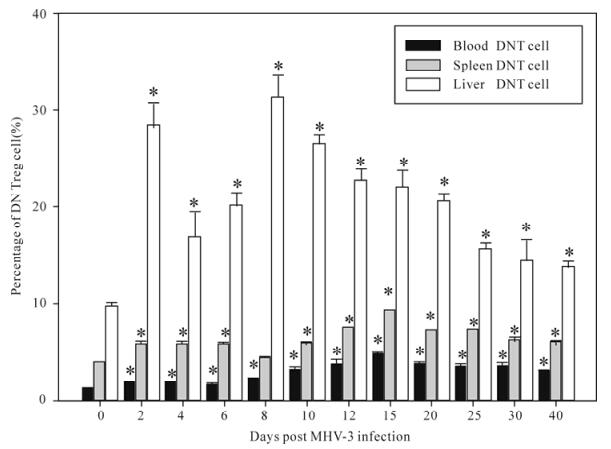
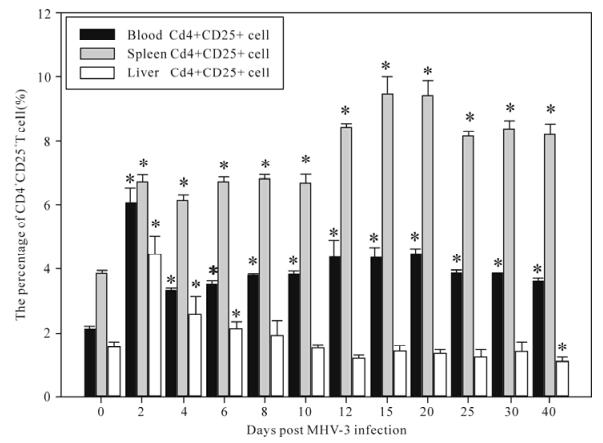
The ratios of T cell subsets of total T lymphocytes of blood, spleen and liver were examined at 0, 2, 4, 6, 8, 10, 12, 15, 20, 25, 30, 40 days post MHV-3 infection through flow cytosorting. We found that the DN Treg cell and CD4+CD25+ T cell ratios of the blood and spleen was raised significantly 2 days post MHV-3 infection in C3H/Hej mice (P < 0.05) (Fig.3 and 4), and the DN Treg cell ratios of the liver raised significantly 2 days post MHV-3 infection (Fig. 1), the CD4+CD25+T cell ratios of the liver was raised significantly at 2, 4, 6, 40 days (P < 0.05) (Fig. 2). CD3+CD4+CD25-, CD3+CD4-CD25+, CD3+CD4+CD8-and CD3+CD4-CD8+ T cell ratios showed a corres-ponding decrease but was not statistically significant (P > 0.05).
Observation of the changes of virus titer and inflammatory in liver
Observation of the ratios of the subsets in T lymphocyte
-
The presented data demonstrates that donor lymphocytes infusion could help recipients to establish specific donor antigen immunological tole-rance before allergenic transplantation (2). DN Treg cells of a recipient could be activated by the infusion of donor lymphocytes. Activated DN Treg cells produced predominantly INF-γand TNF-α, and could eliminate T lymphocytes of specific donor antigens of the recipient in the periphery and organ transplanted. This is of great importance for the permanent survival of the donor allergenic skin grafts and cardiac transplantation (15, 16, 17). In-vitro experiments demonstrated that DN Treg cells had specific cytotoxic effects on syngeneic MHC-antigen complex CD8+ T cells through Fas/FasL interaction after activation (14, 15, 18). Yang et al observed that the ratio of DN Treg cells was raised significantly in response to the aggravation due to the fulminating hepatitis of BalB/C mouse (13). These data show that DN Treg cells may have very important functions in the development of viral hepatitis.
CD4+CD25+ T cell can inhibit the proliferation, activation of CD8+ and CD4+ T lymphocytes and secretion of IL-2, IFN-γ through the secretion of TGF-β, IL-10 (4, 11). A recent study demonstrated that the ratios of CD4+CD25+ T cell in blood of chronic hepatitis B virus (HBV) infected patients was raised significantly more than that in normal control people and convalescent HBV infected patients. In-vitro studies also demonstrated that these CD4+ CD25+ T cells could specifically inhibit HBV antigen specific helper T cells, and induce immunological tolerance and HBV chronic infection (4). The ratio of CD4+CD25+ T cells which were hepatitis C virus antigen specific in blood of chronic hepatitis C virus (HCV) patients was also raised as the disease progressed (11). These reports indicate that CD4+ CD25+ T cells may have important roles in the development of chronic viral hepatitis.
In this study we observed that the virus was titer raised and showed persistent virus duplications and inflammatory changes in the liver 2 days post MHV-3 infection in C3H/Hej mice(Fig.1 and 2). The ratios of DN Treg cells and CD4+CD25+ T cell in the blood and spleen was also raised significantly 2 days post MHV-3 infection in C3H/Hej mice (P < 0.05) (Fig.3 and 4), and the DN Treg cell ratios of the liver was raised significantly 2 days post MHV-3 infection. And the CD4+CD25+ T cell ratio of the liver was raised significantly at 2, 4, 6, 40 days (P < 0.05) (figure 3, 4). CD3+CD4+CD25-, CD3+CD4-CD25+, CD3+CD4+CD8-and CD3+CD4-CD8+ T cell ratios decreased accordingly but this was not statiscally significant (P > 0.05). We postulate that after the infection of MHV-3, the virus duplicated persistently in livers and induced chronic hepatitis, and the ratios of DN Treg cell and CD4+CD25+ T cell in C3H/Hej was raised compen-satedly as the antigen specific cytotoxic T lymphocyte mediated immunologic injury was enhanced. DN Treg cells may inhibit this kind of hyperimmunoreaction through the effect of direct cytotoxicity to the virus specific CD8+ T cells. Also, CD4+CD25+ T cells can inhibit this kind of hyperimmunoreaction through the inhibition of the activation and proliferation of CD4+ T cells, CD8+ T cells, and secretion of IL-2, IFN-γ or the direct cytotoxic effect to the virus specific CD8+ T cell. Above all, our study indicates that DN Treg cells and CD4+CD25+ T cells have suppressive immunomo-dulation functions and may be one of the reasons for persistent viral infection. Further studies will be performed to investigate the function and molecular mechanisms of the immunomodulation of DN Treg cells and CD4+CD25+ T cells.
The present report demonstrates that DN Treg cells and CD4+CD25+ T cells have important immunomo-dulation functions which have important significance in the immune state of infection disease, autoimmune disease, transplantation tolerance and tumor immune responses. Nevertheless, there considerable work is still required to elucidate the roles of the T cells in the regulation of the human diseases. Once these func-tions and mechanisms of the regulatory T cell are clarified, it will be very useful for the development of the future immunotherapy.














 DownLoad:
DownLoad: