HTML
-
Chronic hepatitis B virus (HBV) infection is a serious public health issue affecting more than 250 million individuals (Christian et al. 2014; Schweitzer et al. 2015). Two types of drugs have been approved by the Food and Drug Administration (FDA) for anti-HBV therapy: interferon-α and nucleos(t)ide analogues (Jacquard et al. 2006; Watcharasak et al. 2011; Zhu et al. 2009). However, only one-third of HBV patients are sensitive to interferon-α treatment (Jacquard et al. 2006; Watcharasak et al. 2011; Zhu et al. 2009). Thus, nucleos(t)ide analogues have become the standard of care for HBV infections (Watcharasak et al. 2011; Zoulim and Locarnini 2009). Currently, five nucleos(t)ide analogues, including lamivudine (3TC), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine (LdT), and tenofovir disoproxil fumarate (TDF) are approved by the FDA for anti-HBV therapy (Jacquard et al. 2006; Watcharasak et al. 2011; Zhu et al. 2009; Zoulim and Locarnini 2009). Mechanistic studies demonstrated that nucleos(t)ide analogues inhibit HBV DNA replication by terminating DNA synthesis. However, drug-resistant mutants emerged after long-term treatment with nucleos(t)ide analogues have limited the wide application of these drugs (Guo et al. 2018; Schinazi et al. 2012; Zoulim and Locarnini 2009). For example, Meng et al. (2017) recently reported that the 3TC resistance rate in China had gradually increased from 39.8% in 2010 to 56.6% in 2013 (Zhang et al. 2018). In contrast, other drugs, such as TDF and ETV, possess high genetic barriers and remain effective against most 3TC-resistant mutants. However, it was recently suggested that a few 3TC resistance mutations (rtA181V, rtN236T, rtL180M, rtM204V) confer cross-resistance to other nucleoside analogues, such as ETV, ADV, LdT, and TDF (Guo et al. 2018; Meng et al. 2017; Zhang et al. 2018). For example, 3TC-resistant HBV rtA181V mutations can significantly reduce the antiviral efficacy of ADV, LdT, and TDF (Meng et al. 2017; Zhang et al. 2018). During the past 7 years, the frequency of rtA181V single-base mutations, which is positively correlated with the wide usage of ETV, has increased gradually in China (Zhang et al. 2018). Long-term administration of ADV also resulted in the development of rtN236T mutation. Furthermore, Suzuki et al. (2014) recently reported that TDF therapy may induce the generation of HBV resistance mutants. Although drug resistance is not a serious problem in current clinical practice with ETV, TDF, and TAF treatment, it is necessary to develop novel antiHBV drugs with increased drug-resistant barriers or distinct drug-resistant profiles for use in combination antiviral therapies in the future.
The antiviral activity of 2', 3'-dideoxyguanosine (DoG) against human immunodeficiency virus (HIV) has been examined since 1990 (Busso et al. 1990; Jeffrey et al. 2014). DoG has also been demonstrated to have anti-duck hepatitis B virus (DHBV) efficacy in vivo (Chen et al. 2007) and a favorable safety profile compared to AZT (Zeng et al. 2009; Zhang et al. 2010, 2011). However, its antiviral activity against different hepadnaviruses remains unclear. HepAD38 cells are an ideal anti-HBV drug evaluation model in which HBV replication is controlled by the tetracycline (Tet) suppressor, which has been widely used to evaluate the anti-HBV activity of compounds (Ladner et al. 1997). Dstet 5 is a DHBV replication system from a chicken hepatoma cell line controlled in a tetracyclinedependent manner, which is often used to investigate the mechanism of anti-HBV drugs (Condreav et al. 1990). In the present study, we utilized these two cell culture systems to evaluate the effect of DoG against hepadnaviruses. Furthermore, the antiviral effects of DoG on woodchuck hepatitis virus and HBV-resistant mutants were evaluated by transient transfection assays.
-
LMH cells were cultured in DMEM/F12 medium (Mediatech, Herndon, Virginia, USA) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. HepAD38 is a HepG2-derived stable cell line supporting tetracycline-inducible HBV genome replication (Guo et al. 2007; Ladner et al. 1997). Dstet 5 is an LMH-derived DHBV replication model controlled by tetracycline (Condreav et al. 1990; Guo et al. 2013). Both cell lines were maintained in DMEM/F12 medium. Lamivudine (3TC) was purchased from Sigma (St. Louis, MO, USA). The plasmids pTREHBV (wild-type), pTREHBV/ rtL180V, and pTREHBV/rtN236T were constructed previously (Yu et al. 2011).
-
To evaluate the antiviral effect of DoG on HBV and DHBV, HepAD38 cells or Dstet 5 cells were plated into 12-well plates at a density of 5 × 105 cells/well and cultured in DMEM/F12 media containing 1 μg/mL tetracycline for 2 days. The cells were then mock-treated or treated with different concentrations of DoG for 5 days (HBV) or 3 days (DHBV) without tetracycline. For woodchuck hepatitis virus (WHV), HepG2 cells were seeded into 12-well plates at a density of 8 × 105 cells/well and grown overnight. The cells were then transfected with 1 μg of pCMVWHV per well with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions. Six hours post-transfection, the cells were mock-treated or treated with 3TC or different concentrations of DoG for 5 days. Intracellular viral core DNA and total RNA were extracted and analyzed by Southern blot or Northern blot hybridization with α-32PUTP (800 Ci/mmol, Perkin Elmer, Waltham, MA, USA) labeled plus-strand or minus-strand specific full-length HBV, DHBV, or WHV riboprobe, as described previously (Campagna et al. 2013; Zhang et al. 2016).
-
To investigate the antiviral profile of DoG against different HBV drug-resistant mutants, HepG2 cells were seeded into 24-well plates at a density of 4 × 105 cells per well and cultured in DMEM/F12 overnight. Each well was then transfected with 0.6 μg of pTREHBV-resistant mutants and 0.3 μg of pTet-off (CloneTech, Mountain View, CA, USA) with Lipofectamine 2000 (Invitrogen). Six hours after transfection, the plates were treated with 3TC or different concentrations of DoG for 5 days. HBV DNA or RNA was extracted and analyzed as previously described (Campagna et al. 2013; Zhang et al. 2016).
-
To investigate the molecular mechanism of DoG against HBV, the 3' termini of novel RNA species were mapped as previously described (Zhang et al. 2016). Briefly, HepAD38 cells were exposed to 10 μmol/L of 3TC or 40 μmol/L of DoG for 3 days after removing the tetracycline. The RNA were extracted from cytoplasmic nucleocapsids as described previously (Zhang et al. 2016). After removing the mRNA-containing poly A+ tail with an Oligotex mRNA kit (Qiagen, Hilden, Germany), an artificial poly A+ tail was added to the flow-through RNA with a poly (A) Tailing Kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer's protocol. The 3' termini of HBV RNA were determined by a 3'-RACE procedure as described previously (Zhang et al. 2016).
Cell Lines and Chemicals
Southern Blot or Northern Blot Analysis of Hepadnaviral DNA or RNA
Analysis of Antiviral Activity of DoG against Wild-Type and Drug-Resistant Mutants of HBV
Sequencing of 3' Termini of Novel RNA Species
-
HepAD38 cell line is a tetracycline-inducible HBV producer cell that supports genotype D HBV replication (Guo et al. 2007; Ladner et al. 1997). To evaluate the anti-HBV activity of DoG, HepAD38 cells were exposed to different doses of DoG for 5 days after removing tetracycline. The HBV DNA from viral particles was extracted and analyzed by Southern blot hybridization and quantified as described previously (Campagna et al. 2013; Zhang et al. 2016). As expected, our data indicated that DoG dose-dependently inhibited wild-type HBV replication (Fig. 1). The EC50 of DoG was 0.3 ± 0.05 μmol/L. Moreover, in agreement with its mode of action, DoG did not reduce the levels of intracellular HBV RNA. However, similar to other antiHBV nucleos(t)ide analogues, DoG treatment induced the accumulation of a less than full-length viral RNA species, which was demonstrated recently by our group to be derived from HBV pgRNA (Zhang et al. 2016).
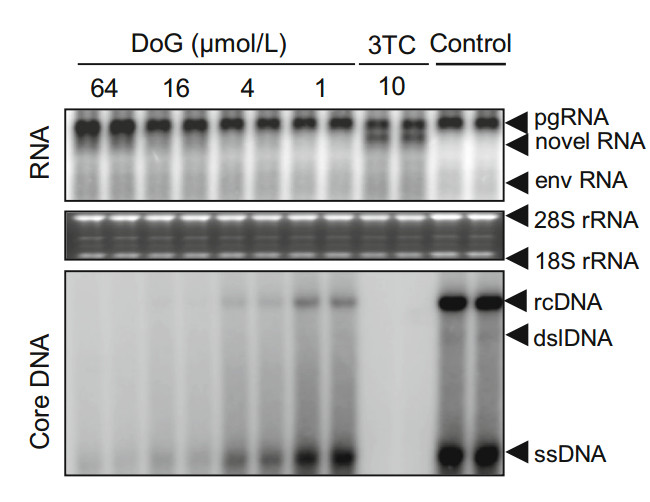
Figure 1. Southern blot and Northern blot analyses of anti-HBV effect of DoG. HepAD38 cells were incubated with DoG in the absence of Tet for 5 days. Northern or Southern blot was conducted to analyze the total RNA and viral core DNA with a HBV-specific probe. Relative HBV DNA level of each group was quantified by Quantity One software (Bio-Rad, Hercules, CA, USA). Representative results from three experiments are presented. 28S, and 18S rRNA, 28S and 18S ribosomal RNA; pgRNA, pregenomic RNA; env RNA, mRNAs encoding envelope proteins; dslDNA, double-stranded linear DNA; rcDNA, relaxed circular DNA; ssDNA, single stranded DNA
-
To investigate the antiviral effect of DoG on DHBV, Dstet 5 cells were incubated with DoG for 3 days after removing tetracycline. As shown in Fig. 2A, DoG dose-dependently reduced DHBV DNA levels. The EC50 value of DoG was 6.82 ± 0.25 μmol/L. As observed in HepAD38 cells, DoG did not alter the levels of intracellular DHBV RNA, but promoted the generation of a novel RNA band derived from RNase H cleavage of pgRNA. Interestingly, similar to observations in the HepAD38 HBV model, the electrophoretic mobility of the novel RNA species induced by DoG treatment in the DHBV model was also slightly slower than that of RNA species caused by 3TC, suggesting that DoG arrests hepadnaviral minus-stranded DNA synthesis at an earlier stage (Fig. 2A). To further investigate whether the novel RNA species induced by DoG in Dstet 5 can also be generated from viral pgRNA transcribed from covalently closed circular (ccc) DNA, we designed an experimental procedure based on a previously described method (Zhang et al. 2016). Briefly, Dstet 5 cells were cultured without tetracycline for 3 days to allow the synthesis of cccDNA. Next Dstet 5 cells were incubated in media with tetracycline to block transgene transcription. Thereafter, Dstet 5 cells were exposed to DoG for additional 3 days and viral RNA was extracted and analyzed as previously described (Zhang et al. 2016). As expected, DoG treatment resulted in accumulation of similar novel RNA species as 3TC treatment under the same experimental conditions (Fig. 2B), suggesting that this RNA species can be generated from the pgRNA transcribed from either the DHBV transgene or cccDNA.
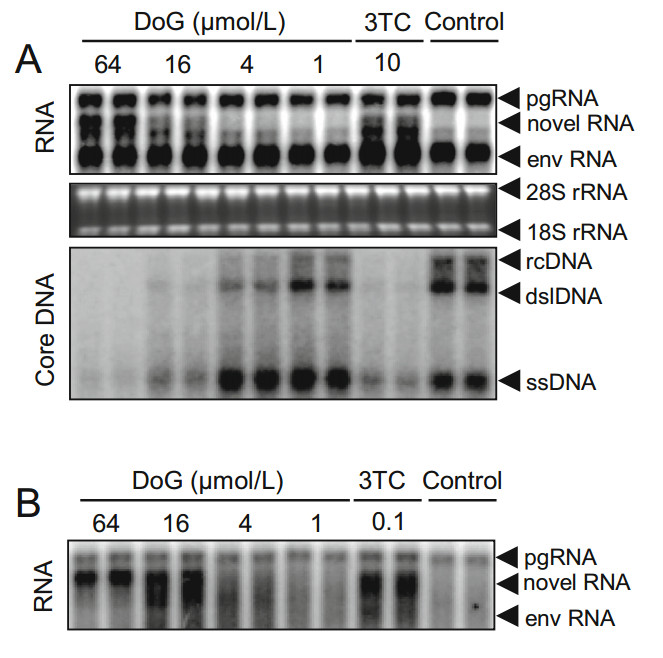
Figure 2. Southern blot and Northern blot analyses of antiviral effect of DoG on DHBV. Dstet 5 cells were exposed to DoG for 3 days. A Total RNA and DHBV DNA were examined by Northern or Southern blot with DHBV-specific probes. Relative HBV DNA level in each group was quantified with Quantity One software (Bio-Rad). Ribosomal RNAs served as loading controls. B The core particles were extracted as previously described (Zhang et al. 2016). Capsidassociated RNA was analyzed by Northern blotting. Representative results are presented from three experiments. 28S, and 18S rRNA, 28S and 18S ribosomal RNA; pgRNA, pregenomic RNA; env RNA, mRNAs encoding envelope proteins; dslDNA, double-stranded linear DNA; rcDNA, relaxed circular DNA; ssDNA, single stranded DNA
-
WHV represents a well-characterized mammalian hepadnavirus infection model that is widely used to evaluate the anti-HBV efficacy of candidate drugs (Menne and Cote 2007; Korba et al. 2000; Menne et al. 2008). To investigate whether DoG inhibits the replication of WHV, HepG2 cells were transfected with pCMVWHV plasmid and incubated with the indicated concentrations of DoG or 3TC for 5 days. Intracellular WHV DNA replicative intermediates were examined by Southern blotting as described previously (Campagna et al. 2013; Zhang et al. 2016), and quantified with a phosphorimager. As shown in Fig. 3, DoG dose-dependently inhibited WHV replication. The EC50 value of DoG on intracellular WHV DNA synthesis was 23.0 ± 1.5 μmol/L.
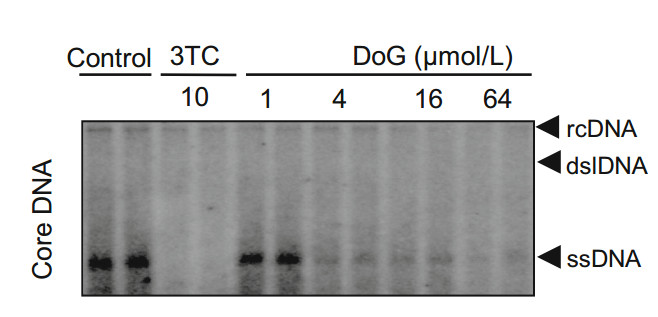
Figure 3. Southern analysis of antiviral effect of DoG on WHV. pCMVWHV plasmids were transfected into HepG2 cells with Lipofectamine 2000. At 6 h post-transfection, the cells were incubated with DoG and 10 μmol/L 3TC for 3 days. Core DNA was examined by Southern blotting. Relative HBV DNA level in each group was quantified by Quantity One software (Bio-Rad). Representative results from three experiments are presented. rcDNA, relaxed circular; dslDNA, doublestranded linear DNA; ssDNA, single-stranded viral DNA
-
Long-term therapy with nucleos(t)ide analogs for chronic hepatitis B may result in the development of drug-resistant HBV variants (Meng et al. 2017). To explore the relative antiviral potency of DoG towards clinically prevalent HBV drug-resistant variants, HepG2 cells were transiently transfected with plasmids containing HBV mutants rtA181V and rtN236T resistant to 3TC and adefovir, respectively) (Korba et al. 2000; Menne and Cote 2007). After 5 days of treatment with DoG, HBV DNA from viral particles was examined by Southern blotting or quantitative polymerase chain reaction (qPCR) as previously described (Campagna et al. 2013; Zhang et al. 2016). As shown in Fig. 4, DoG markedly inhibited the viral DNA synthesis of the rtA181V 3TC-resistant mutant (Fig. 4A, EC50- = 0.89 ± 0.22 μmol/L) and rtN236T adefovir-resistant mutant (Fig. 4B, EC50 = 0.66 ± 0.08 μmol/L).
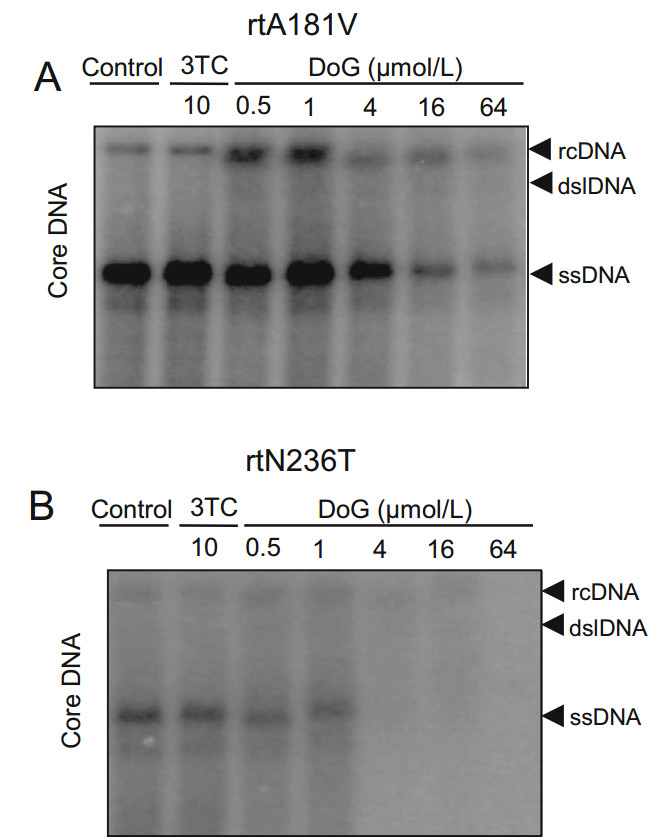
Figure 4. Southern blot analysis of antiviral effect of DoG on mutant HBV. HepG2 cells transfected with rtA181V (A), or rtN236T (B). HBV mutant plasmids were treated with DoG and 10 μmol/L3TC for 3 days. Viral core DNA was detected by Southern blot. Viral core DNA was quantified by Quantity One software (Bio-Rad) and the relative percentages compared to the negative control are presented. Representative results from two experiments are presented. rcDNA, relaxed circular; dslDNA, double-stranded linear DNA; ssDNA, single-stranded viral DNA
-
To evaluate the 3' end of the novel RNA species induced by DoG treatment, HepAD38 cells were incubated with 3TC or DoG for 3 days. The viral RNA was extracted, and the full-length HBV pgRNA was removed by using an oligo (dT)-containing adaptor as previously described (Zhang et al. 2016). The flow-through RNA species were then artificially added to a poly-A tail and then reversetranscribed into cDNA as previously described (Zhang et al. 2016). HBV RNA were amplified by PCR and the PCR products were cloned and sequenced as previously described (Zhang et al. 2016). Our results indicated that the 3' end of the viral RNAs produced following DoG treatment were mainly at nt 1650-1820 of the pgRNA. However, consistent with our previous finding, the 3'-end of the viral RNA in 3TC treated cells were mapped to nt 1400–1780 (Fig. 5).
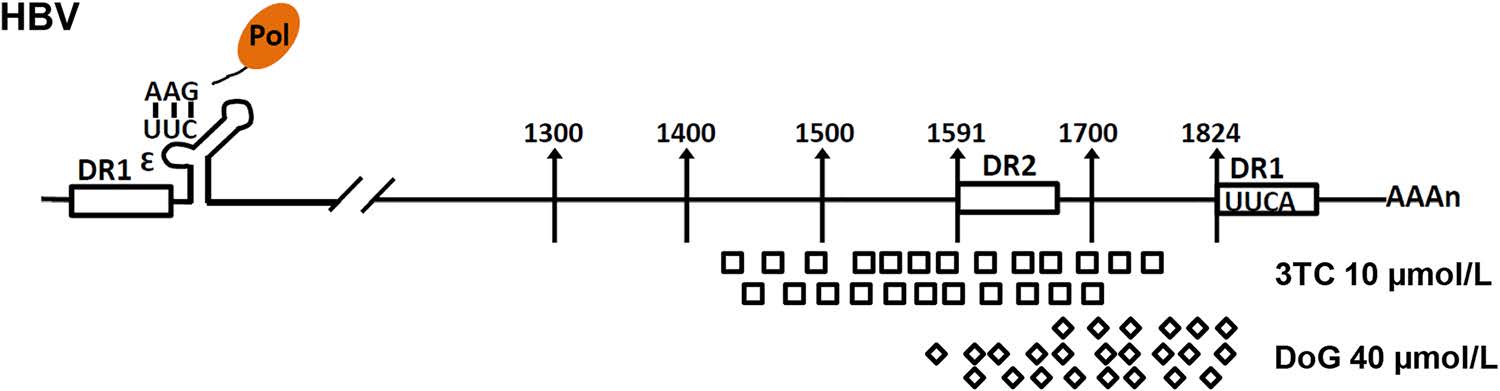
Figure 5. Sequencing of 3' ends of encapsidated viral RNA caused by DoG. HepAD38 cells were treated with 10 μmol/L 3TC or 40 μmol/L DoG for 3 days. The poly-free viral RNA was collected, their 3' termini were determined by a 3'-RACE procedure as previously described (Zhang et al. 2016). The positions of the 3' termini of these novel RNA species were confirmed by sequencing
Anti-HBV Activity of DoG in HepAD38 Cell Line
Anti-DHBV Activity of DoG in Dstet 5 Cell Line
Anti-WHV Effect of DoG
Antiviral Activity of DoG on HBV Mutants
Sequencing the 3' End of Novel RNA Species
-
In this present study, we determined the antiviral properties of DoG on different hepadnaviruses (HBV, DHBV, or WHV) and clinically prevalent drug-resistant HBV variants. Our results demonstrated that DoG significantly inhibited the replication of HBV, DHBV, and WHV in cell culture models. We also confirmed similar in vitro antiviral effects of DoG on wild-type and the clinically relevant HBV drug-resistant strains that encode rtA181V and rtN236T mutations. These results indicate that DoG is useful as a hepadnaviral polymerase inhibitor.
Nucleos(t)ide analogues are the only category of directacting antivirals approved by the FDA for chronic hepatitis B therapy (Schinazi et al. 2012; Zoulim and Locarnini 2009). In vitro biochemical studies showed that nucleos(t)ide analogs act as chain terminators of viral DNA synthesis (Jones et al. 2013; Staschke and Colacino 1994). Liu et al. (2013) recently revealed that the existence of additional DHBV RNA species in Dstet 5 cells treated with 3TC. Our recent study demonstrated that these specific RNAs are derived from RNase H cleavage of encapsidated pgRNA during reverse transcriptional synthesis of minus strand DNA (Zhang et al. 2016). Coincidently, HBV RNA species caused by nucleos(t)ide analogues treatment in HBV patients was recently confirmed to be from encapsidated pgRNA in virus-like particles (Wang et al. 2016). Consistent with our observations in vitro, ETV therapy significantly reduced the HBV DNA load in the serum, but did not reduced the amount of serum HBV RNA, suggesting that the action mechanism of nucleoside analogues is mainly to inhibit the reverse transcription of HBV pgRNA. Furthermore, HBV RNA species in serum may predict viral rebound after the termination of nucleoside analog therapy. Interestingly, our sequencing results revealed that the termination of DHBV minus-strand DNA synthesis in vivo caused by nucleos(t)ide analogues such as 3TC and ETV mainly occurred at the 3' end of DHBV pgRNA (between nt 1909 and 2522), suggesting that the synthesis of minus strand viral DNA terminates at the early reverse-transcription step (Zhang et al. 2016). Furthermore, we found that the length of this novel RNA species was positively associated with the antiviral efficacy of nucleoside analogs. For example, compared to 3TC, the length of novel RNA species caused by ETV was longer than those of 3TC treatment, suggesting that ETV more efficiently inhibited viral DNA synthesis at an early step of reverse transcription.
To investigate the intrinsic mechanism of termination of hepadnaviral DNA reverse transcription by DoG, we first determined the effect of DoG on the pgRNA of HBV in HepAD38 cells (Condreav et al. 1990; Ladner et al. 1997). As observed following treatment with 3TC, DoG treatment also dose-dependently resulted in the production of specific RNA species in HepAD38 cells. Interestingly, with increasing of DoG concentrations, this RNA band gradually narrowed into a smaller band (Fig. 1), suggesting that the transcription of minus-strand DNA was nearly completely blocked by DoG. Our sequencing results also further confirmed that the 3' termini of novel RNA species induced by DoG treatment were mainly limited to a more narrow region of pgRNA (nt 1650–1820) compared to those after 3TC treatment (nt 1400–1780), suggesting that reverse transcription starting from the 3' termini of HBV pgRNA was more efficiently terminated by DoG.
Similar to observations in the HepAD38 cells, compared to 3TC, the specific RNA species produced following DoG treatment in Dstet 5 cells migrated slightly slower than those after 3TC treatment (Fig. 2A), suggesting that DoG more efficiently suppresses DHBV minus-stranded DNA synthesis at the initial steps. Paradoxically, however, the effect of DoG on HBV or DHBV DNA replication appeared to be significantly weaker than that of 3TC. To further evaluate this difference, the pharmacokinetics of DoG in Sprague–Dawley rats was analyzed by liquid chromatography mass spectrometry. The pharmacokinetic profiles of DoG at 50 mg/kg in rats indicated that DoG was rapidly absorbed and reached a maximum concentration (Cmax) in a short time of 3–5 min, suggesting that the weaker effect of DoG on inhibiting HBV replication than that of 3TC was related to the rapid metabolism of DoG in vivo (Li et al. 2008). Therefore, reducing the in vivo metabolic rate through structural modification or dosage formulation changes should be examined in future clinical trials.
Therapy with nucleoside analogues, such as 3TC, adefovir, entecavir, telbivudine, and tenofovir, is highly effective against HBV. However, their therapeutic value may be limited by the emergence of HBV-resistant mutants (Guo et al. 2018; Schinazi et al. 2012). For example, the single rtA181T mutation in HBV polymerase confers cross-resistance to 3TC, ADV, and LdT (Ahn et al. 2014). Although ADV remains relatively sensitive to 3TCresistant HBV mutants, ADV-resistant mutants remain more prevalent because of the widespread use of ADV monotherapy (European Association for the Study of the Liver 2012; Liaw et al. 2012). Mutations in rtA181V/T/S or rtN236T were found to be mainly associated with ADV resistance (Chinese Society of Hepatology and Chinese Society of Infectious Diseases 2011; Liaw et al. 2012). Therefore, we further compared the susceptibility of 3TCor ADV-resistant HBV mutants to DoG. As shown in Fig. 4, DoG effectively suppressed the replication of an HBV mutant harboring ADV-resistant rtA181V or rtN236T, suggesting that DoG is more suitable for 3TC- or ADV-resistant strains. However, whether DoG alone or combination with adefovir is an effective antiviral therapy in cases of adefovir resistance requires further examination.
In summary, we demonstrated that DoG significantly inhibited the replication of DHBV, HBV, and WHV. Compared to 3TC, DoG more efficiently inhibited the minus-strand viral DNA synthesis of HBV or DHBV by terminating elongation of the DNA chain. Interestingly, DoG effectively suppressed the replication of an HBV mutant harboring 3TC-resistant rtA181V or ADV-resistant rtN236T. Our in vitro data provide information for improving the anti-HBV activity of DoG in future study.
-
The work was supported in part by the Program for New Century Excellent Talents in University (Program No. NCET-12-0975); by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry; and by Jiangsu Overseas research & training program for University Prominent Young and Middle-aged Teachers and Presidents (for Pinghu Zhang); Qinghai Province High-end Innovative Talent Thousand Talents Program (for Pinghu Zhang).
-
PZ mainly contributed to the design, acquisition, analysis, and interpretation of data for this work; ZS contributed to the sequencing of viral RNA species; JC and JTG contributed to the experimental design and data interpretation of this work.
Acknowledgements
Author Contributions
-
The authors declare that they have no conflict of interest.
-
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.















 DownLoad:
DownLoad: