-
Dear Editor,
In December 2019, SARS-CoV-2 was first detected in the samples obtained from three adult patients who suffered from an unknown viral pneumonia in Wuhan (Li et al. 2020). This unknown viral pneumonia is further named as coronavirus disease 2019 (COVID-19) by the World Health Organization. To date, the number of new COVID-19 cases has continued to skyrocket and the impact of SARS-CoV-2 on humans is far greater than any pathogen of this century in both breadth and depth. Previous studies have shown that adults with COVID-19 have symptoms of fever, dry cough, dyspnea, fatigue and lymphocytopenia. Moreover, COVID-19 is more likely to cause death in the elderly, especially those with chronic comorbidities (Huang et al. 2020). In Wuhan, more than 50, 000 COVID-19 cases have been confirmed, including over 780 pediatric patients, and only one child death case (Lu et al. 2020). Although the number of children cases was far fewer than that of adults, COVID-19 might endanger children's health and the information on children remains limited, especially in serological study. In the retrospective study, the investigators analyzed the epidemiological, clinical and serological characteristics of children with COVID-19 in Wuhan in the early stages of the outbreak, which might provide theoretical and practical help in controlling COVID-19 and similar emerging infectious diseases in the future.
The retrospective study was conducted at Women and Children's Hospital of Hubei Province (Wuhan, China), a designated hospital providing medical services to children with confirmed and suspected COVID-19 during the outbreak. Initially, we enrolled 56 suspected inpatients with COVID-19 from 25 January 2020 to 2 March 2020. Fourteen inpatients with viral pneumonia were positive for SARS-CoV-2 nucleic acid test (named group NP) and diagnosed as confirmed cases with COVID-19. Another 20 suspected inpatients with negative SARS-CoV-2 nucleic acid result were detected by in-house anti-SARS-related coronaviruses IgM and IgG ELISA kits in Wuhan Institute of Virology and positive for the antibody (named group AP) (Supplementary Fig. S1). The ELISA kits had been deployed to detect IgM and IgG in adult patients with COVID-19 in Wuhan during the outbreak and worked well (Zhang et al. 2020; Zhou et al. 2020). It was the first time of the ELISA kits to be used to detect SARS-CoV-2 antibody in children.
In group NP, most patients were tested positive for viral nucleic acid within 12 days after onset, a small number of patients still tested positive from 18 to 30 days after onset (Fig. 1A). It is suggested that viral shedding of SARS-CoV-2 may persist for a long time. In addition, 6 of the 14 patients in group NP were also positive for the antibody (Table 1).
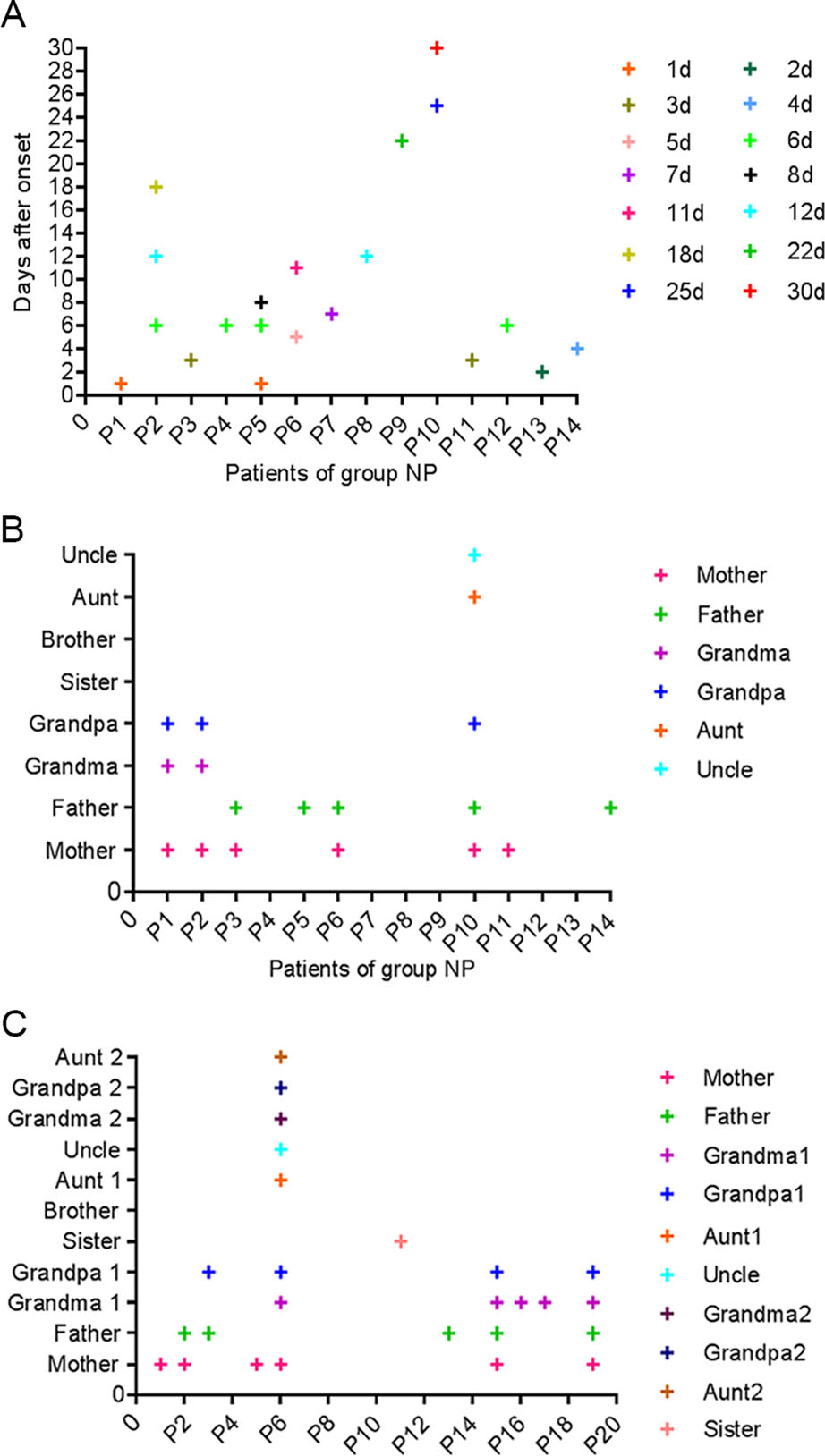
Figure 1. SARS-CoV-2 nucleic acid test in group NP and familial cluster infection in group NP and AP. A Fourteen patients in group NP were positive for SARS-CoV-2 nucleic acid. B Eight patients in group NP had a contact history, such as parents, grandparents or relatives who were positive for SARS-CoV-2 nucleic acid. C Eleven patients in group AP had a contact history, such as parents, grandparents, sister or relatives who were positive for SARS-CoV-2 nucleic acid.
Characteristic Total (n = 34) Group NP (n = 14) Group AP (n = 20) SARS-CoV-2 RNA, No (%) 14 (41.2) 14/14 (100.0)* 0* SARS-CoV-2 antibody, No (%) Ig M (+) and / or IgG (+) 26 (100.0) 6/6 (100.0) 20 (100.0) Only Ig M (+) 3 (11.5) 1/6 (16.7) 2 (10.0) Only IgG (+) 16 (61.5) 3/6 (50.0)* 13 (65.0)* IgM (+) and Ig G (+) 7 (26.9) 2/6 (33.3) 5 (25.0) *Refers to a statistically significant difference between group NP and group AP, P < 0.05. Table 1. SARS-CoV-2 nucleic acid and antibody detection of 34 patients.
In group NP and AP, the main age distribution were within 0–2 years old and 6–10 years old, and 10 (71.4%) and 14 (70%) patients were male (Table 2). It suggested that strengthening the protective isolation of infants, toddlers and school-aged children was very important. Compared to girls, boys were more frequently affected. The same dominance of male patients was also observed in adult patients with COVID-19, SARS and MERS (Badawi and Ryoo 2016; Channappanavar et al. 2017; Chen G et al. 2020; Chen N et al. 2020; Huang et al. 2020). The increase in male susceptibility to coronavirus infections could be correlated to Y chromosome and sex hormones, which may be involved in innate and adaptive immunity (Guo et al. 2019; Chen G et al. 2020; Chen N et al. 2020).
Characteristic Total (n = 34) Group NP (n = 14) Group AP (n = 20) Age, y, median (IQR) 5.0 (1.0–9.0) 5.6 (1.1–9.0) 4.5 (0.9–9.4) Age distribution, No. (%) 0–1 years 8 (23.5) 3 (21.5) 5 (25.0) 1–2 year 8 (23.5) 2 (14.3) 6 (30.0) 2–6 years 3 (8.8) 1 (7.1) 2 (10.0) 6–10 years 10 (29.4) 7 (50.0) 3 (15.0) > 10 years 5 (14.7) 1 (7.1) 4 (20.0) Gender, No. (%) Female 10 (29.4) 4 (28. 6) 6 (30.0) Male 24 (70.6) 10 (71.4) 14 (70.0) Familial cluster, No. (%) 19 (55.9) 8 (57.1) 11 (55.0) Breast feeding, No. (%) (< 1y) 
6/8 (75.0) 3/3 (100.0) 3/5 (60.0) Coexisting disorder, No. (%) 1 (2.9) 0 1 (5.0) Clinical classification Common 30 (88.2) 13 (92.9) 17 (85.0) Severe 4 (11.8) 1 (7.1) 3 (15.0) Time from illness onset to hospital, ds, median (IQR) 15.8 (9.0–19.3) 18.4 (9.8–24.3) 14.0 (9.0–16.3) Symptoms and signs, No. (%) Fever 24 (70.6) 10 (71.4) 14 (70.0) Cough 28 (82.4) 13 (92.6) 15 (75.0) Sputum production 15 (44.1) 6 (42.9) 9 (45.0) Shortness of breath 4 (11.8) 1 (7.1) 3 (15.0) Cyanosis 1 (2.9) 1 (7.1) 0 Sore throat 3 (8.8) 1 (7.1) 2 (10.0) Running nose 8 (23.5) 4 (28.6) 4 (20.0) Vomiting 4 (11.8) 0 4 (15.0) Diarrhea 2 (5.9) 1 (7.1) 1 (5.0) Blood routine, (normal; × 109/L), median (IQR) Leukocyte count, (5.5–12.0) 8.2 (6.1–9.7) 6.1 (4.9–7.7)* 9.7 (6.7–11.5)* Neutrophil count, (1.1–3.9) 4.5 (2.1–5.5) 2.9 (1.4–4.4)* 5.6 (3.1–7.8)* Lymphocyte count, (1.2–6.0) 3.0 (1.5–4.2) 2.5 (1.4–3.1) 3.3 (1.8–4.6) < 1.2, No. (%) 4 (11.8) 1 (7.1) 3 (15.0) 1.2–6.0, No. (%) 26 (76.5) 12 (85.7) 14 (70.0) > 6.0, No. (%) 4 (11.8) 1 (7.1) 3 (15.0) Platelet count, (100–300) 255 (158–325) 252 (144–300) 257 (165–333) Infection-related biomarkers (normal range), median (IQR) C-reactive protein, (0–10.0 mg/L) 9.4 (0.3–11.6) 3.7 (0–8.5) 13.5 (2.0–15.4) > 10, No. (%) 9 (26.5) 3 (21.4) 6 (30.0) Procalcitonin, (< 0.05 ng/mL)a 0.7(0.1–0.9) 0.1 0. 8 (0.1–0.9) > 0.05, No. (%) 11 (78.6) 1/1 (100.0) 10 / 13 (76.9) Humoral immunity, (normal; g/L), median (IQR)b IgA, (0.14–2.7) 1.0 (0.2–1.3) 1.3 (0.27–2.2) 0.7 (0.17–1.24) IgM, (0.15–2.6) 1.0 (0.6–1.3) 1.0 (0.5–1.6) 0.9 (0.7–1.3) IgG, (3.0–16.5) 7.8 (6.4–10.9) 8.1 (5.2–11.6) 7.6 (6.5–10.5) Liver and kidney function, (normal range), median (IQR) ALT, (5–40 U/L) 23.0 (13.4–27.3) 22.8 (12.4–28.4) 23.0 (14.9–27.5) AST, (8–40 U/L) 35.5 (25.6–43.0) 35.1 (25.0–44.1) 35.8 (26.1–43.7) TB, (1.71–17.1 μmol/L) 6.1 (3.4–7.9) 5.8(3.5–7.1) 6.3(3.2–9.1) BUN, (2.9–7.1 mmol/L) 3.7 (2.9–4.5) 3.6(3.1–4.6) 3.7 (2.8–4.5) CRE, (30–106 μmol/L) 32.0 (22.0–42.0) 35.25 (24.1–47.8) 30.4 (22.0–36.5) Co-infection, No. (%) Mycoplasma—IgG (≥ 1:160) 14 (41.2) 5 (35.7) 9 (45.0) Influenza virus A-RNA (+) 1 (2.9) 0 1 (5.0) Respiratory syncytial virus-RNA (+) 6 (17.6) 4 (28.6) 2 (10.0) Epstein-Barr virus-DNA (+) 1 (2.9) 1 (7.1) 0 Sputum culture for streptococcus pneumonia 1 (2.9) 1 (7.1) 0 Computed tomography, No. (%) Ground-glass opacity 14 (41.2) 6 (42.8) 8 (40.0) Local patchy shadowing 25 (73.5) 10 (71.4) 15 (75.0) Bilateral patchy shadowing 16 (47.1) 6 (42.8) 10 (50.0) Right lung patchy shadowing 24 (70.6) 11 (78.6) 13 (65.0) Treatment, No. (%) Antiviral therapy 24 (70.6) 13 (92.9)* 11 (55.0)* Antibiotic therapy 34 (100.0) 14 (100.0) 20 (100.0) Immunoglobin 1 (2.9) 0 1 (5.0) Corticosteroid 2 (5.8) 1 (7.1) 1 (5.0) Chinese patent drug 16 (47.1) 9 (64.3) 7 (35.0) Oxygen support 4 (11.8) 1 (7.1) 3 (15.0) Prognosis, No. (%) Discharge 34 (100.0) 14 (100.0) 20 (100.0) Hospital stays, ds, median (IQR) 9.4 (7.0–12.0) 11.2 (6.8–16.3) 8.2 (7.0–8.8) Group NP, confirmed cases with SARS-CoV-2 nucleic acid positive. Group AP, cases with SARS-CoV-2 antibody positive. IQR, interquartile range; Ig, immunoglobulin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; BUN, blood urea nitrogen; CRE, creatinine; RNA, ribonucleic Acid; DNA, deoxyribonucleic acid; +, positive.

*Refers to a statistically significant difference between group NP and group AP, P < 0.05.
aProcalcitonin was available from 1 and 13 patients in the two groups.
bIgA, IgM and IgG were available from 9 and 11 patients in the two groups.Table 2. Epidemiological and clinical characteristics of 34 patients.
In group NP and AP, the 8 and 11 patients caught SARS-CoV-2 through their parents, grandparents, sister or relatives with SARS-CoV-2 nucleic acid positive, respectively (Fig. 1A and 1C). It suggested that children with COVID-19 mainly occurred in familial cluster, when compared to adults with COVID-19, who acquired infection mainly through communities, families, or hospitals (Chan et al. 2020; Chen G et al. 2020; Chen N et al. 2020; Cui et al. 2020; Huang et al. 2020; Su et al. 2020; Wang D et al. 2020; Wang XF et al. 2020). In both groups, 6 out of 8 (75%) infants were breastfed (Table 2). Since we did not concurrently perform SARS-CoV-2 nucleic acid in breast milk, it was not clear whether the infants get infected by breastfeeding and (or) respiratory transmission of their mothers.
In group NP and AP, the common symptoms were fever (10, 71.4%; 14, 70%) and cough (13, 92.6%; 15, 75%), merely 1 (7.1%) and 3 (15%) patients developed shortness of breath, as did the proportion of patients with lymphocytopenia. The incidence of severe cases in children was significantly lower than that in adults (Chen G et al. 2020; Chen N et al. 2020; Huang et al. 2020; Wang D et al. 2020; Wang XF et al. 2020). Lymphocytopenia was not common in children, it is true for another study (Wang D et al. 2020; Wang XF et al. 2020). In adult patients, lymphocytopenia is an important reference index for COVID-19 diagnosis and for the disease severity (Chen G et al. 2020; Chen N et al. 2020; Zhu et al. 2020). Thus, in children, the absence of lymphocytopenia is a remarkable difference. The average neutrophil count in group NP was lower than that in group AP (P < 0.05). It seemed to be negatively correlated with the higher level of viral nucleic acid in group NP. In both groups, 5 (35.7%) and 9 (45%) patients were co-infected with mycoplasma, 4 (28.6%) and 2 (10%) patients presented with respiratory syncytial virus (RSV) co-infection (Table 2).
In group NP, 6 (42.8%) patients had ground-glass opacity, 10 (71.4%) patients had local patchy shadowing, 6 (42.8%) patients had bilateral patchy shadowing, and 11 (78.6%) patients had right lung patchy shadowing on chest computed tomography (CT) scans (Supplementary Fig. S2). In group AP, there were 8 (40%), 15 (75%), 10 (50%) and 13 (65%) patients for each of four category imaging changes (Table 2). However, ground-glass opacities and bilateral lesions were observed in 60% and 75%–100% adult patients, respectively (Chen G et al. 2020; Chen N et al. 2020; Huang et al. 2020; Wang D et al. 2020; Wang XF et al. 2020; Xia et al. 2020). Overall, lung inflammation in children was less severe than in adults.
Of the 26 patients with SARS-CoV-2 antibody positive in both groups, 3 (11.5%), 16 (61.5%), and 7(26.9%) patients were positive for only IgM, only IgG, and both IgM and IgG, respectively (Table 1). IgM and IgG appeared at 1–9 days and 2–22 days after onset, respectively. In adults with COVID-19, the IgM and IgG were detected on 3–6 days and 10–18 days after onset (Guo et al. 2020). Thus, the specific antibody response to SARS-CoV-2 in children appeared to be earlier than in adults. In addition, the positive ratio of IgG in our study was 88.5%, slightly higher than that of the adults (77.9%), while IgM was 38.5%, obviously lower than that of the adults (85.4%) (Guo et al. 2020). The possible reason was that they took twice or three times blood samples per adult patient, whereas we took only one sample per child. For individuals, the specific antibody may not reach the detection threshold during the window period of serological conversion. Thus, repeated detection of SARS-CoV-2 antibodies at different time can further improve the positive rate of serological detection. Interestingly, a 2-month-old baby had high levels of IgM and IgG on 7 days after onset (Fig. 2A). The baby's mother was a confirmed case with COVID-19. So far, the investigators could not completely rule out the following two possibilities. First, the baby was infected by means of breast milk and (or) respiratory tract transmission. Second, the baby's antibodies were self-produced and (or) the maternal antibody transfer through breastfeeding. Some small sample studies showed that the positive detection rate of SARS-COV-2 nucleic acid in breast milk was not high, and no infectious virus particles were isolated in breast milk with SARS-CoV-2 nucleic acid positive, but the composition of numerous breast milk proteins and metabolites changed significantly (Chambers et al. 2020; Groß et al. 2020; Zhao et al. 2020). The mother-to-child transmission of SARS-CoV-2 still needs a large-sample multi-center joint study to clarify.
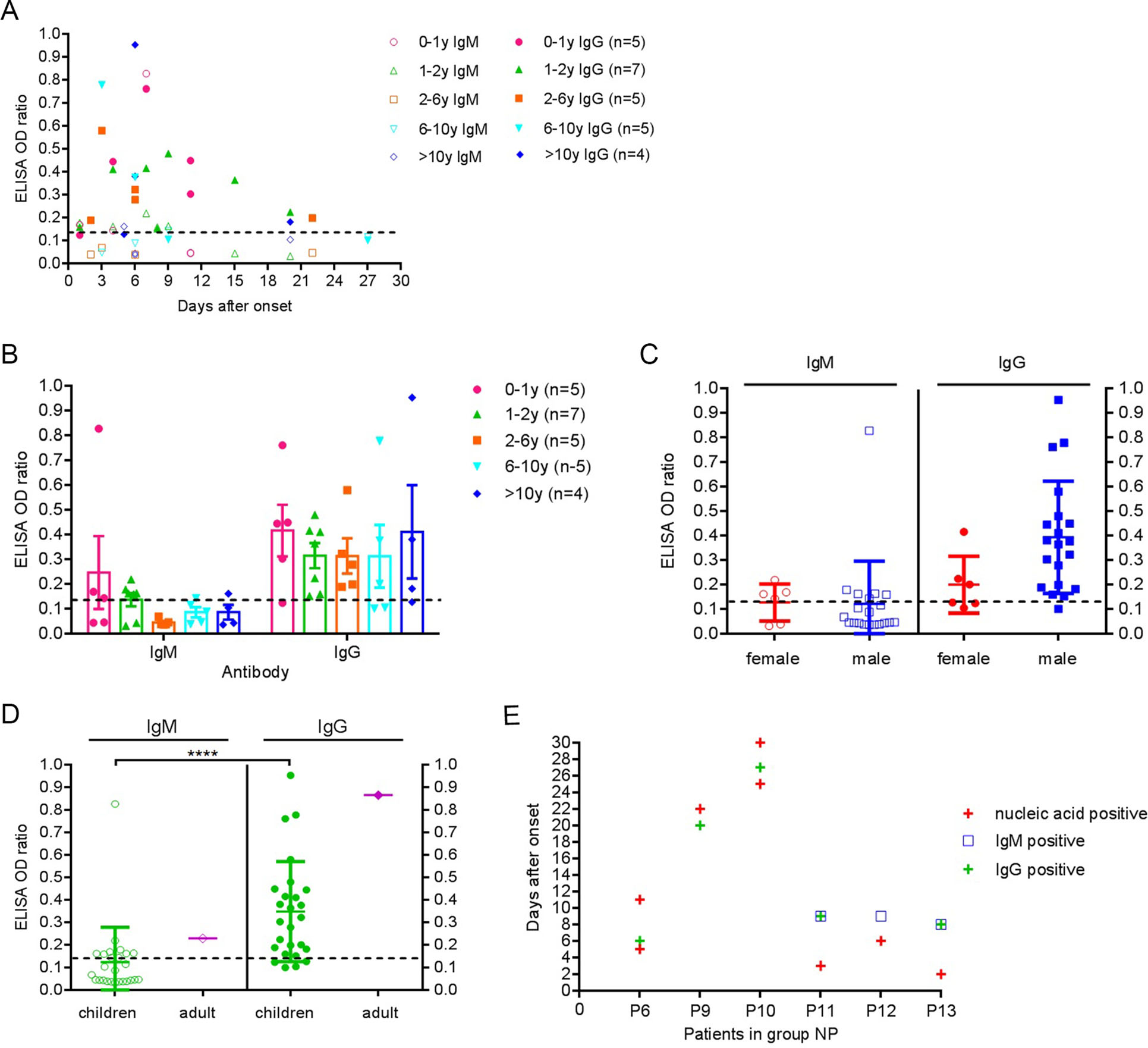
Figure 2. SARS-CoV-2 antibody of 26 children patients in group NP and AP. A SARS-CoV-2 IgM and IgG of 26 children patients. B SARS-CoV-2 IgM and IgG levels at different age (P > 0.05). C SARS-CoV-2 IgM and IgG levels at different gender (P > 0.05). D The level of SARS-CoV-2 IgM and IgG of 26 children and an adult. E SARS-CoV-2 nucleic acid and antibody detection of 6 patients in group NP. The dashed line indicates the cutoff value.
No significant differences were detected in the mean level of IgM and IgG at different age (P > 0.05) (Fig. 2B), the same was true for SARS-CoV-2 antibody levels in different genders (P > 0.05) (Fig. 2C). At present, there are no serological studies with age and gender as evaluation indicators of COVID-19. In addition, the specific IgM and IgG level in individual children were comparable to that of a convalescent adult with COVID-19 (Fig. 2D).
In group NP, two patients were positive for IgM and IgG at 8–9 days after onset. More than 20 days after onset, the other two patients were still positive for viral nucleic acids and IgG (Fig. 2E). Comprehensive analysis of SARS-CoV-2 antibody showed that the duration of the specific IgM positive was short, and the duration of the IgG positive was relatively long. Thus, specific IgG detection can also be used in epidemiology studies in large samples (Zhang et al. 2020).
Empirical antiviral therapy was administered to 13 (92.9%) in group NP and 11 (55.0%) patients in group AP, respectively (P < 0.05). As of 14 March 2020, all patients were recovered and discharged. From the results above, except for the neutrophil count, SARS-CoV-2 nucleic acid and specific IgG, and antiviral therapy, there were no significant differences between group NP and AP.
There were some limitations in the study. First, merely inpatients were enrolled, and outpatients who were asymptomatic or milder were not included. Second, SARS-CoV-2 nucleic acid test and the specific antibody detection of synchronous breast milk were not performed. Third, merely a single blood sample from each patient was collected, and the investigators were unable to monitor the kinetic changes in the specific antibody class and titer.
In summary, COVID-19 in children had its own distinct epidemiological, clinical and serological characteristics, informing that protective isolation of infants, toddlers and school-aged children is critical, especially for boys in families. Lymphocytopenia should not be considered as a routine diagnostic criterion in children with COVID-19. Children with COVID-19 were more likely to be co-infected with mycoplasma. COVID-19 should be screened at the time of diagnosis in children with fever, cough and CT findings of lung involvement. The detection of SARS-CoV-2 antibodies within 1–3 weeks after onset was of great value in diagnosing COVID-19, especially for SARS-CoV-2 nucleic acid false negative. Mother-to-child transmission of infant cases with COVID-19 requires in-depth study.
HTML
-
This study was supported by the Novel Pneumonia Emergency Science and Technology Project of Hubei Province (2020FCA004) and Youth Innovation Promotion Association of Chinese Academy of Sciences (2016302). We thank all patients and their families involved in the study. We also thank Hubei Provincial Center for Disease Control and Prevention.
-
The authors declare that they have no conflict of interest.
-
This study was approved by the Institutional Ethics Board of Women and Children’s Hospital of Hubei Province (No. 2020IECLW019). In view of the urgency to contain this outbreak, a written informed consent was exempted. Instead, an oral consent was obtained from the patient’s family.

















 DownLoad:
DownLoad: