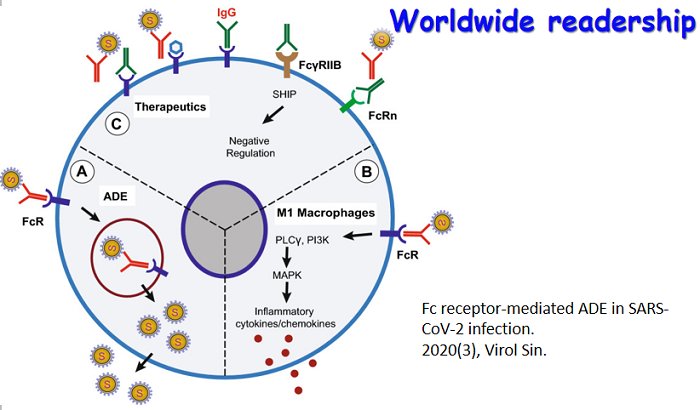
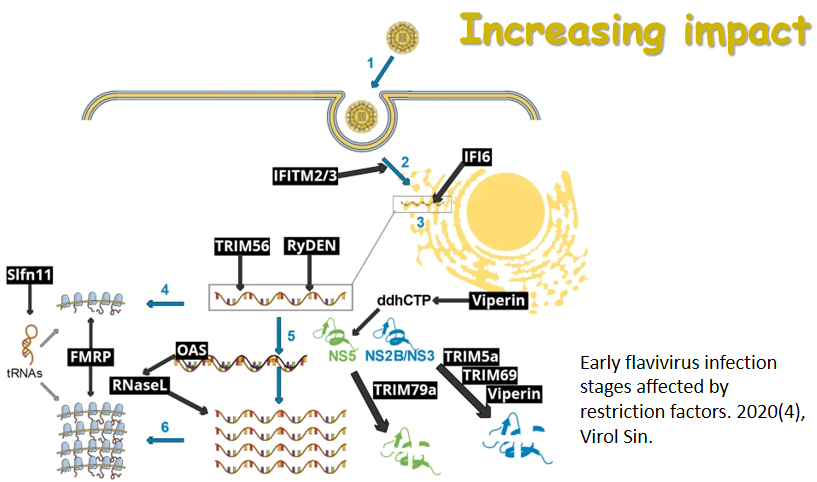
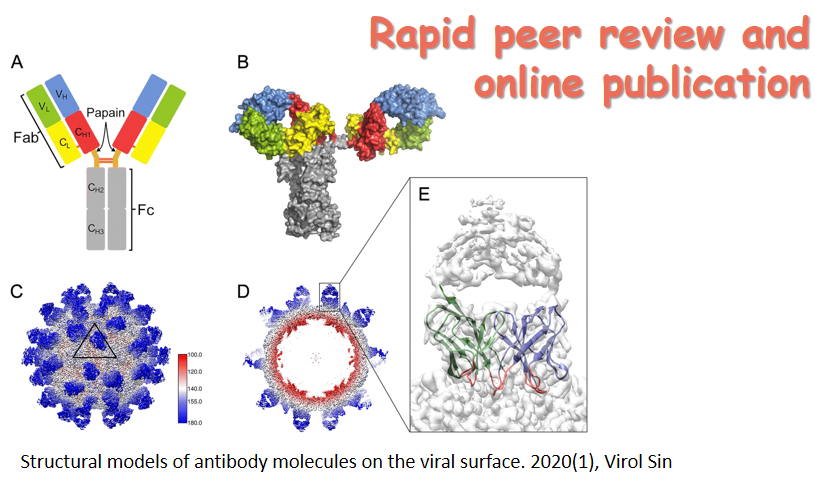
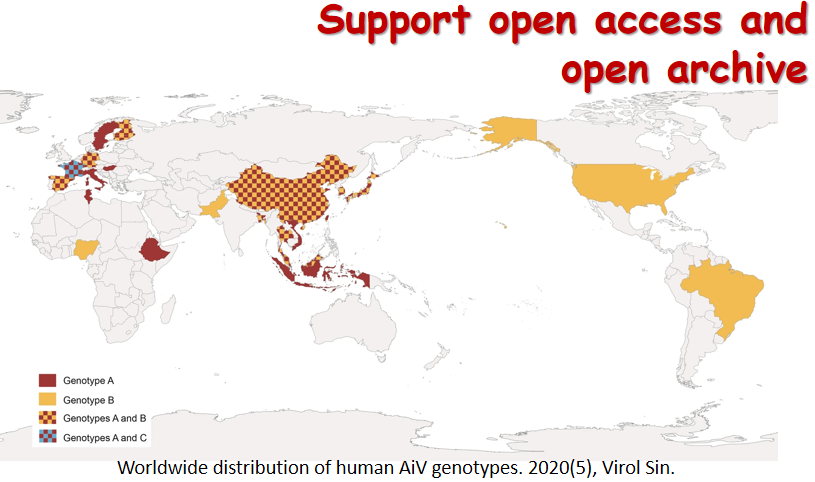
By the end of November 2020, SARS-CoV-2—the new coronavirus behind the disease COVID-19—has infected over 60 million people around the world and caused about one and a half million deaths. Facing the biggest global pandemic of the century, doctors, scientists and the scientific community have been working hard to uncover the pathogenesis and search for effective scientific solutions. Virologica Sinica has online published a series of original articles and reviews on SARS-CoV-2 and COVID-19, covering topics on clinical cohorts/cases and disease features, virus characterization and surveillance, diagnosis and improved methods, antiviral agents and therapeutic treatment, and etc, and they are collectively presented in this special issue. Those researches have greatly advanced our understandings of and empowered our strength to combat the disease. The cover depicts the SARS-CoV-2 virus particle, surrounded by human blood cells.
-
Correction to: Dynamic Changes of Antibodies to SARS-CoV-2 in COVID-19 Patients at Early Stage of Outbreak
2020, 35(6): 887 doi: 10.1007/s12250-020-00318-y
Published: 24 November 2020The coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, has spread around the world with high mortality. To diagnose promptly and accurately is the vital step to effectively control its pandemic. Dynamic characteristics of SARS-CoV-2-specific antibodies which are important for diagnosis of infection have not been fully demonstrated. In this retrospective, single-center, observational study, we enrolled the initial 131 confirmed cases of COVID-19 at Jin-Yin-Tan Hospital who had at least one-time antibody tested during their hospitalization. The dynamic changes of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein in 226 serum samples were detected by ELISA. The sensitivities of IgM and IgG ELISA detection were analyzed. Result showed that the sensitivity of the IgG ELISA detection (92.5%) was significantly higher than that of the IgM (70.8%) (P < 0.001). The meantimes of seroconversion for IgM and IgG were 6 days and 3 days, respectively. The IgM and IgG antibody levels peaked at around 18 days and 23 days, and then IgM fell to below the baseline level at about day 36, whereas IgG maintained at a relatively high level. In conclusion, antibodies should be detected to aid in diagnosis of COVID-19 infection. IgG could be a sensitive indicator for retrospective diagnosis and contact tracing, while IgM could be an indicator of early infection.
-
Correction to: Discrimination of False Negative Results in RT-PCR Detection of SARS-CoV-2 RNAs in Clinical Specimens by Using an Internal Reference
2020, 35(6): 885 doi: 10.1007/s12250-020-00293-4
Published: 21 September 2020Reverse transcription-polymerase chain reaction (RT-PCR) is an essential method for specific diagnosis of SARS-CoV-2 infection. Unfortunately, false negative test results are often reported. In this study, we attempted to determine the principal causes leading to false negative results of RT-PCR detection of SARS-CoV-2 RNAs in respiratory tract specimens. Multiple sputum and throat swab specimens from 161 confirmed COVID-19 patients were tested with a commercial fluorescent RT-PCR kit targeting the ORF1ab and N regions of SARS-CoV-2 genome. The RNA level of a cellular housekeeping gene ribonuclease P/MRP subunit p30 (RPP30) in these specimens was also assessed by RT-PCR. Data for a total of 1052 samples were retrospectively re-analyzed and a strong association between positive results in SARS-CoV-2 RNA tests and high level of RPP30 RNA in respiratory tract specimens was revealed. By using the ROC-AUC analysis, we identified Ct cutoff values for RPP30 RT-PCR which predicted false negative results for SARS-CoV-2 RT-PCR with high sensitivity (95.03%–95.26%) and specificity (83.72%–98.55%) for respective combination of specimen type and amplification reaction. Using these Ct cutoff values, false negative results could be reliably identified. Therefore, the presence of cellular materials, likely infected host cells, are essential for correct SARS-CoV-2 RNA detection by RT-PCR in patient specimens. RPP30 could serve as an indicator for cellular content, or a surrogate indicator for specimen quality. In addition, our results demonstrated that false negativity accounted for a vast majority of contradicting results in SARS-CoV-2 RNA test by RT-PCR. -

Coping with COVID-19 in Sub-Saharan Africa: What Might the Future Hold?
2020, 35(6): 875 doi: 10.1007/s12250-020-00279-2
Received: 04 June 2020 Accepted: 24 July 2020 Published: 01 September 2020Sub-Saharan countries are sadly linked with similar poor indicators, such as high poverty and mortality rates, the burden of disease, fragile health systems and poorly developed infrastructure. Along with the rest of the world, Sub-Saharan countries are facing this new coronavirus outbreak. Nevertheless, chaotic predictions of a particularly destructive epidemic in Africa do not seem to be borne out, at least for the time being. But uncertainties remain, such as how the virus is spreading in countries with low incomes, informal economies, high HIV/tuberculosis prevalence, extremely low median age, or warm/dry climates and for which containments are almost impossible to enforce? Not even 8 months after the first reported case in China, parts of the world are already showing post-lockdown twilight measures. Yet, the war is certainly far from over, because the virus is gaining ground in the sub-Saharan zone. This viewpoint attempts to describe the COVID-19 crisis in a sub-Saharan perspective, in particular in the Republic of Chad, from both, distant perception and by living it on a daily basis. -
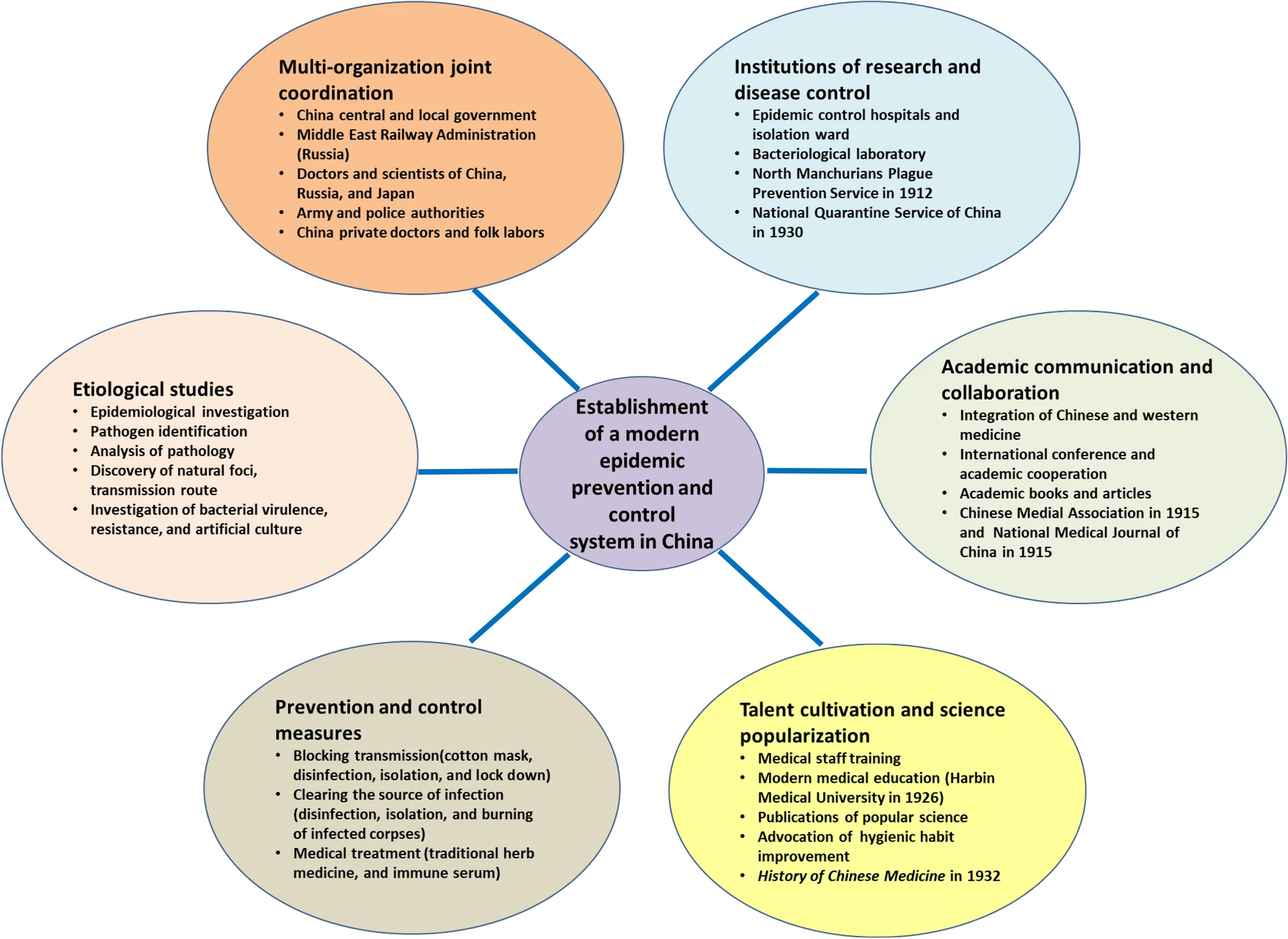
Inception of the Modern Public Health System in China and Perspectives for Effective Control of Emerging Infectious Diseases: In Commemoration of the 140th Anniversary of the Birth of the Plague Fighter Dr. Wu Lien-Teh
2020, 35(6): 868 doi: 10.1007/s12250-020-00269-4
Received: 30 January 2020 Accepted: 28 June 2020 Published: 03 August 2020In this article, we systematically review Dr. Wu Lien-Teh's academic achievements and outstanding contributions in the prevention and control of the plague epidemic in northeast China and introduce the development of the earliest public health epidemic prevention system in China in order to commemorate the 140th anniversary of Dr. Wu Lien-Teh's birth. We hope that this article will provide insights into the effective prevention and control of emerging infectious diseases as well as the current worldwide pandemic of COVID-19, facilitating the improvement and development of public health systems in China and around the globe. -
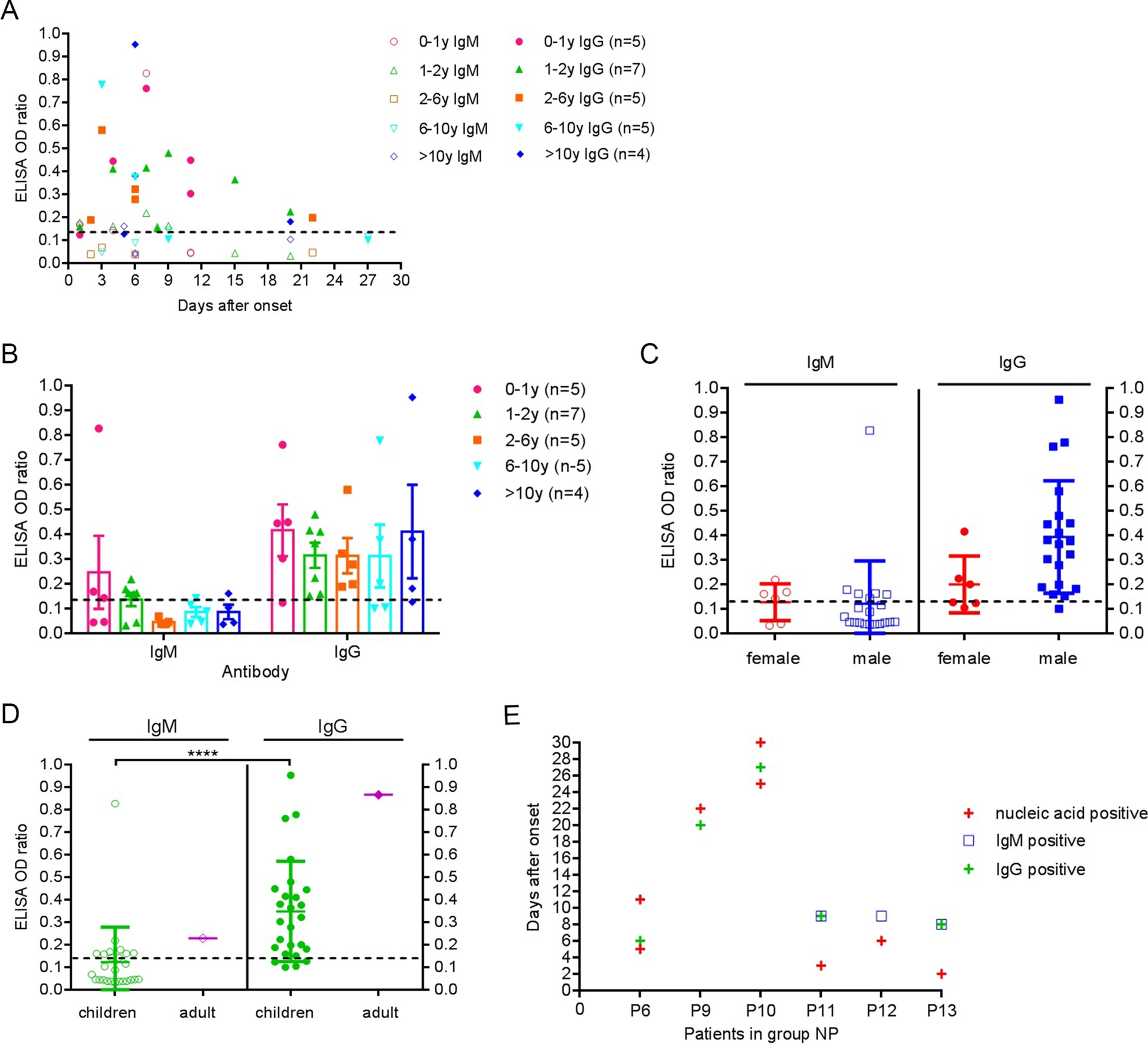
Epidemiological, Clinical and Serological Characteristics of Children with Coronavirus Disease 2019 in Wuhan: A Single-centered, Retrospective Study
2020, 35(6): 861 doi: 10.1007/s12250-020-00333-z
Received: 26 August 2020 Accepted: 30 November 2020 Published: 22 December 2020In December 2019, SARS-CoV-2 was first detected in the samples obtained from three adult patients who suffered from an unknown viral pneumonia in Wuhan (Li et al. 2020). This unknown viral pneumonia is further named as coronavirus disease 2019 (COVID-19) by the World Health Organization. To date, the number of new COVID-19 cases has continued to skyrocket and the impact of SARS-CoV-2 on humans is far greater than any pathogen of this century in both breadth and depth. Previous studies have shown that adults with COVID-19 have symptoms of fever, dry cough, dyspnea, fatigue and lymphocytopenia. Moreover, COVID-19 is more likely to cause death in the elderly, especially those with chronic comorbidities (Huang et al. 2020). In Wuhan, more than 50, 000 COVID-19 cases have been confirmed, including over 780 pediatric patients, and only one child death case (Lu et al. 2020). Although the number of children cases was far fewer than that of adults, COVID-19 might endanger children's health and the information on children remains limited, especially in serological study. In the retrospective study, the investigators analyzed the epidemiological, clinical and serological characteristics of children with COVID-19 in Wuhan in the early stages of the outbreak, which might provide theoretical and practical help in controlling COVID-19 and similar emerging infectious diseases in the future. -

Griffithsin with A Broad-Spectrum Antiviral Activity by Binding Glycans in Viral Glycoprotein Exhibits Strong Synergistic Effect in Combination with A Pan-Coronavirus Fusion Inhibitor Targeting SARS-CoV-2 Spike S2 Subunit
2020, 35(6): 857 doi: 10.1007/s12250-020-00305-3
Received: 12 August 2020 Accepted: 10 September 2020 Published: 14 October 2020In this study, we tested the in vitro inhibitory activity of griffithsin (GRFT) against infection of pseudotyped and live SARS-CoV-2 infection, in order to repurpose the application of GRFT as a potential prophylactic or therapeutic to prevent or treat COVID-19. -
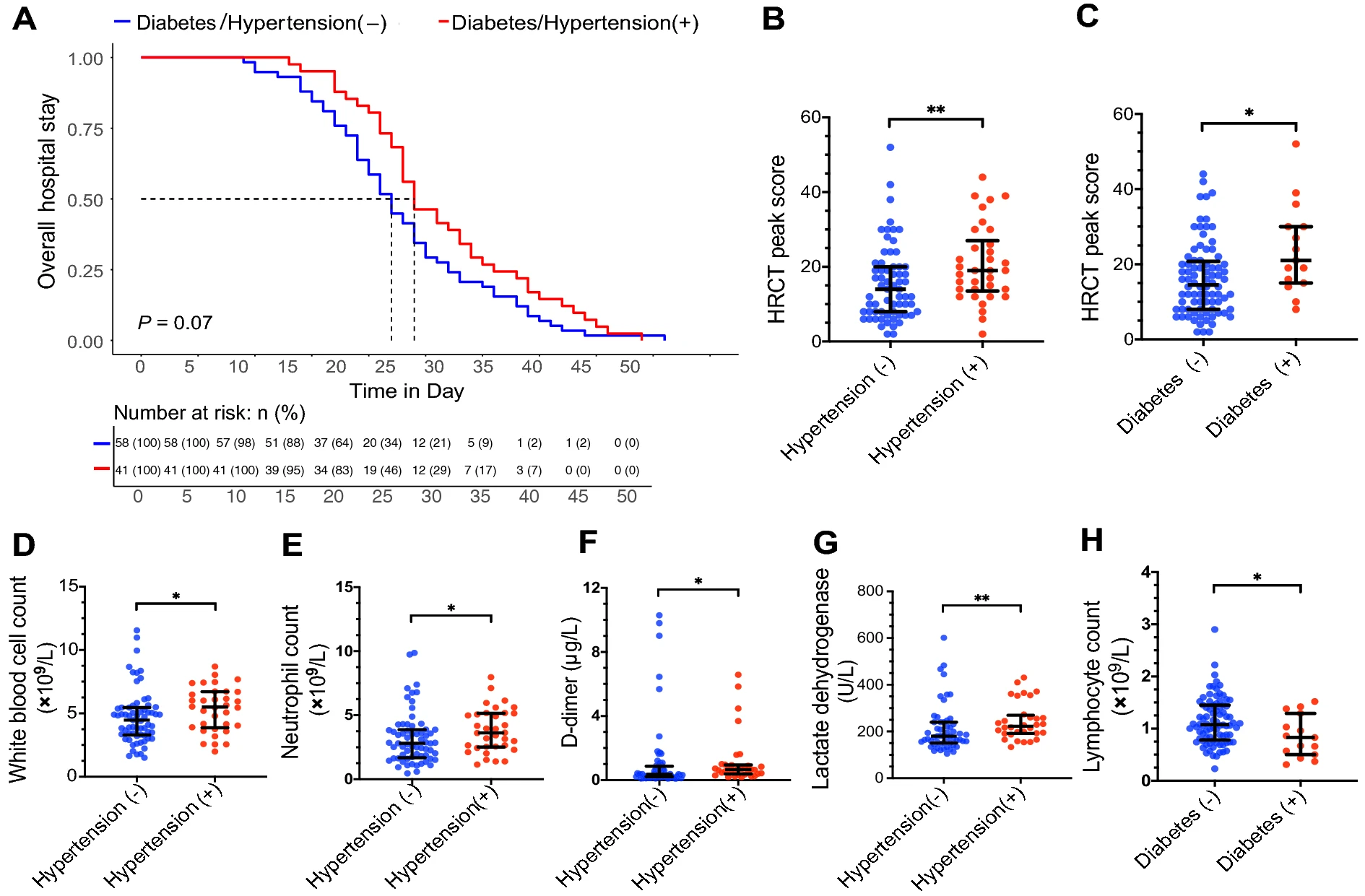
Different Laboratory Abnormalities in COVID-19 Patients with Hypertension or Diabetes
2020, 35(6): 853 doi: 10.1007/s12250-020-00296-1
Received: 09 July 2020 Accepted: 31 August 2020 Published: 30 September 2020We reported recently that hypertension is a risk factor for severe cases of COVID-19, independent of age and other variables (Liu et al. 2020a). An important question is why patients with hypertension and diabetes yield poorer clinical outcomes than those without. Human pathogenic coronavirus SARS-CoV-2 utilizes angiotensin-converting enzyme 2 (ACE2) as a receptor for viral cell entry. Since the levels of ACE2 are substantially increased in patients with hypertension or diabetes, who are treated with ACE inhibitors (ACEIs) and angiotensin Ⅱ type-Ⅰ receptor blockers (ARBs) (Ferrario et al. 2005), Fang and colleagues hypothesized that ACE2-stimulating drugs could potentially increase the risk of developing severe COVID-19 (Fang et al. 2020). This was not supported by a recent study led by Dr. Reynolds (Reynolds et al. 2020), whose analysis showed no positive association for ACEIs or ARBs for either the risk of SARS-CoV-2 infection or severe illness (Reynolds et al. 2020). What else might explain the poorer clinical outcomes of COVID-19 patients with hypertension or diabetes?To explore this question, we re-analysed the same cohort of 99 COVID-19 patients discharged from the general wards of Renmin Hospital of Wuhan University between 5 February 2020 and 14 March 2020 (Ethics approval No: WDRY2020-K124) (Liu et al. 2020a, b). -
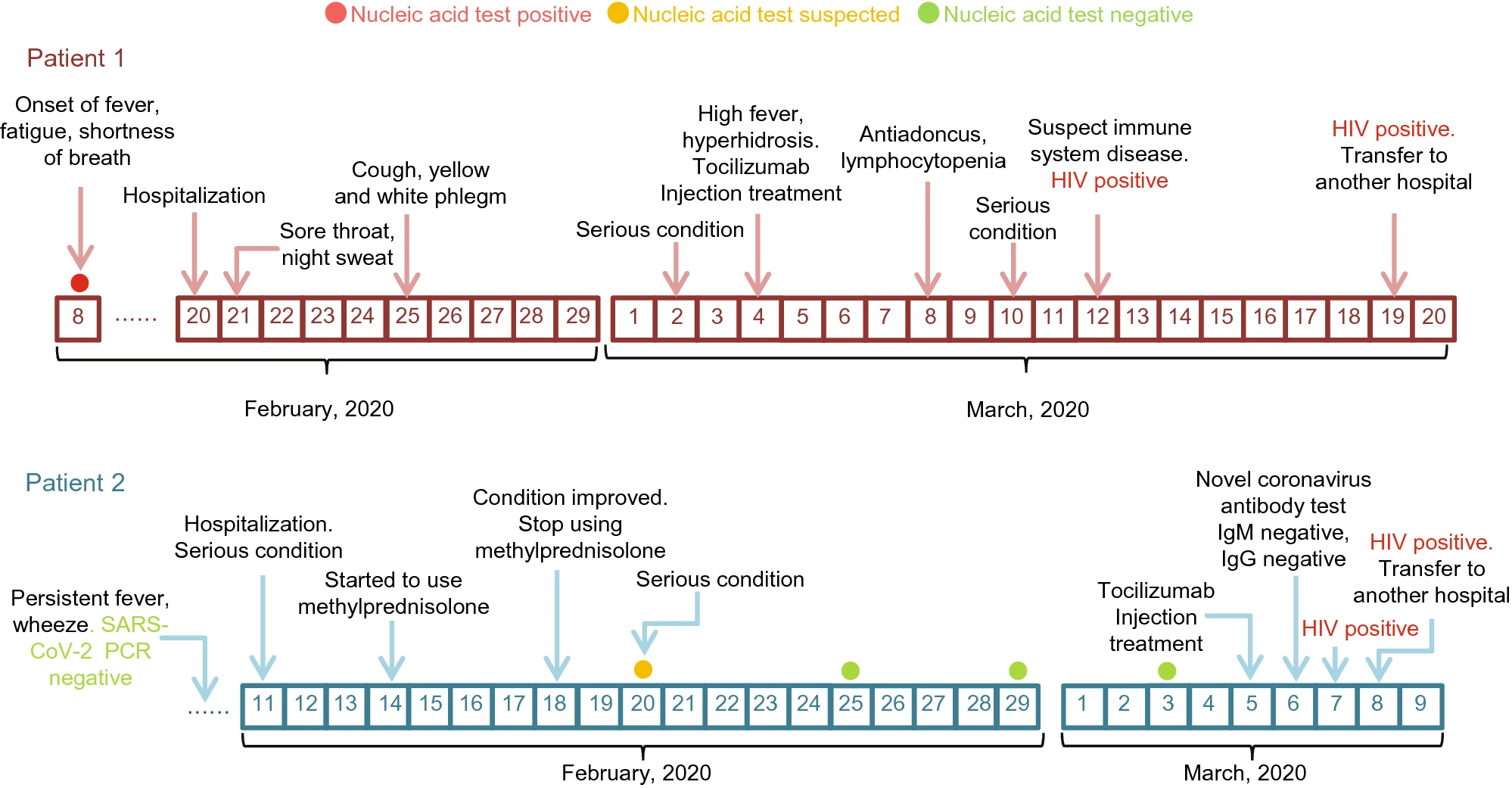
A Study of Two Cases Co-Infected with SARS-CoV-2 and Human Immunodeficiency Virus
2020, 35(6): 849 doi: 10.1007/s12250-020-00280-9
Received: 07 May 2020 Accepted: 13 August 2020 Published: 07 September 2020Since December 2019, A new type of coronavirus pneumonia (coronavirus disease 2019, COVID-19) has become endemic in Wuhan, China. So far, COVID-19 has developed into a global epidemic. The body's immune system plays an important role in the fight against COVID-19. Here, we followed up the clinical data and treatment of two COVID-19 patients diagnosed with acquired immunodeficiency syndrome (AIDS), hoping to be helpful for the subsequent diagnosis and treatment of patients with related diseases. -
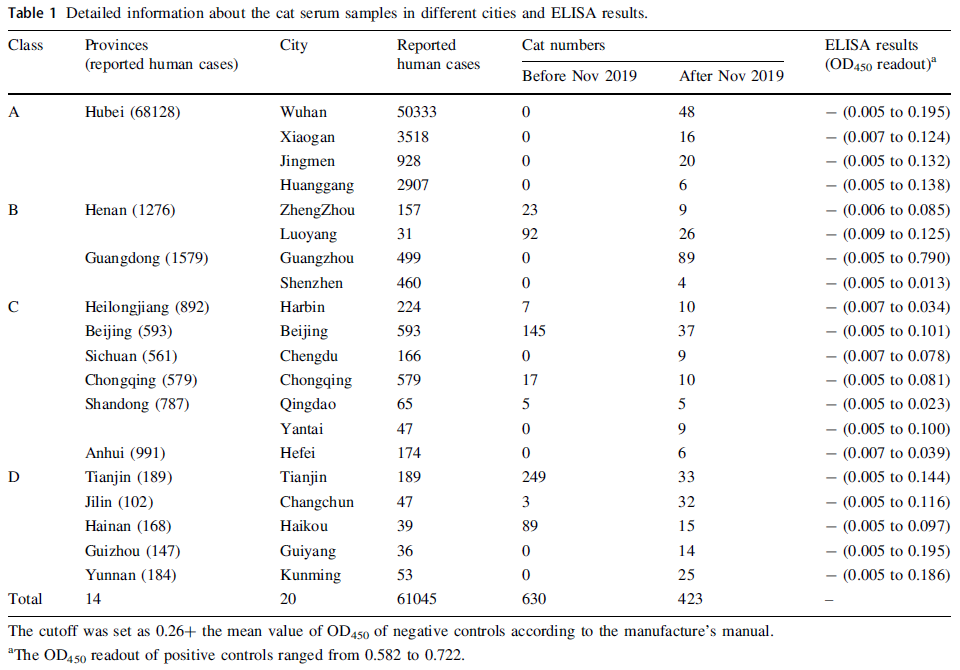
SARS-CoV-2 Serological Survey of Cats in China before and after the Pandemic
2020, 35(6): 846 doi: 10.1007/s12250-020-00284-5
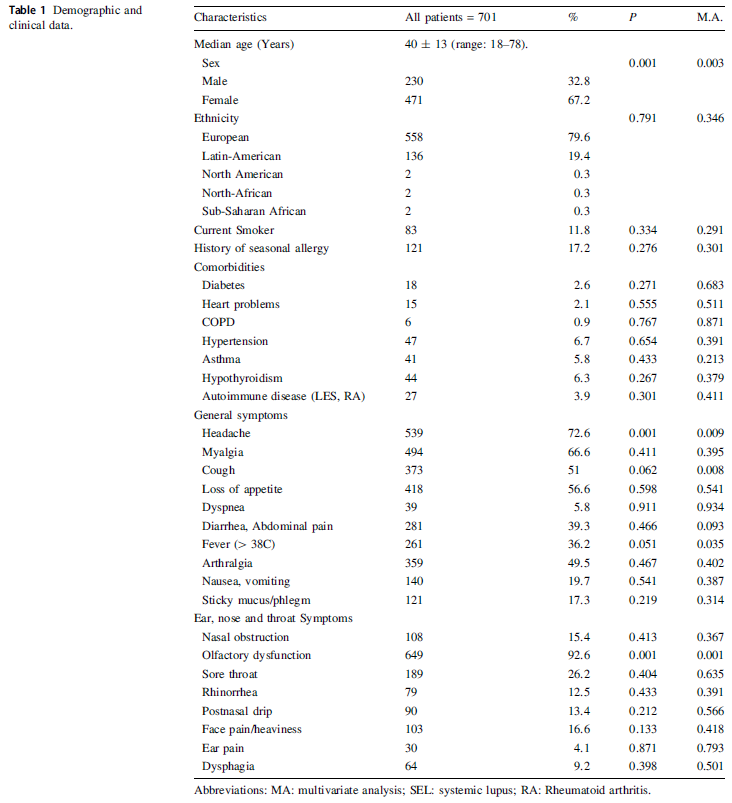
Received: 12 June 2020 Accepted: 03 August 2020 Published: 01 September 2020The high percentage of seropositivity of cats in Wuhan, the epicentre of the SARS-CoV-2 epidemic, could be due to the large number of infected human cases (more than 14 folds than that in other cities) (Table 1) where cats may have been more frequently exposed to SARS-CoV-2 patients or contaminated environment. As yet, SARS-CoV-2 serological prevalence of cats in other Chinese cities remains unknown. Therefore, a serological survey including more cities with numbers of SARS-CoV-2 human cases in China will be valuable for elucidating the role of cats in transmission of the viruses and relieve public concerns. In this study, 630 cat serum samples collected before November 2019 and 423 cat serum samples collected during SARS-CoV-2 outbreak (from February 2020 to April 2020) in 20 cities in China for detecting the prevalence of SARS-CoV-2 specific antibodies. -
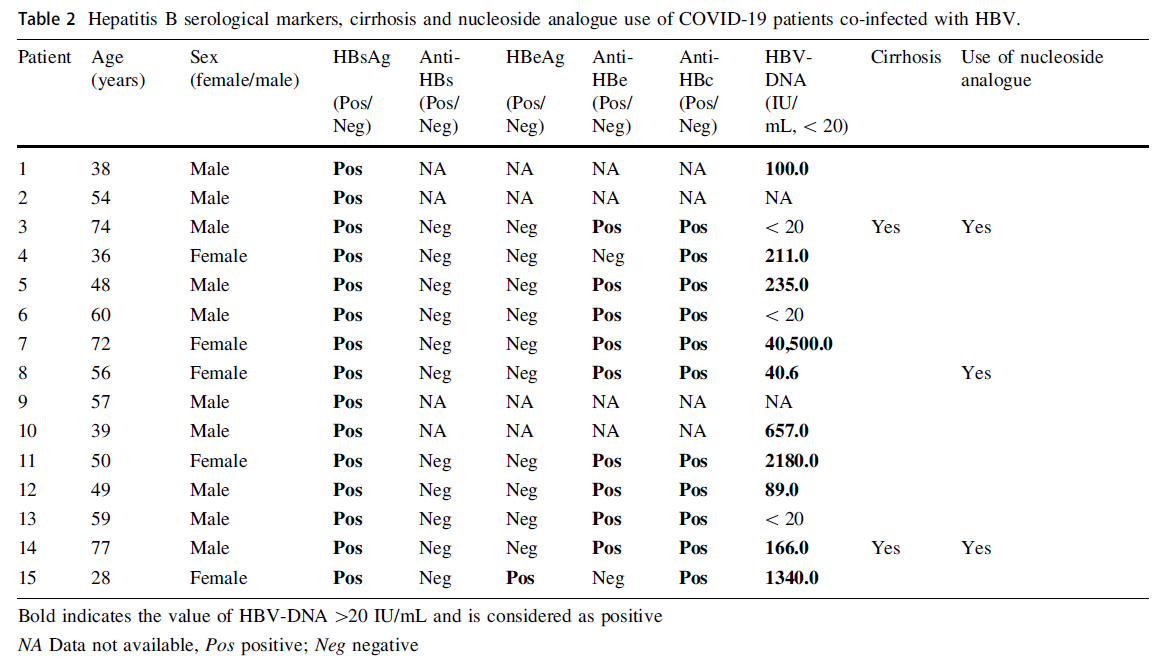
Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection
2020, 35(6): 842 doi: 10.1007/s12250-020-00276-5
Received: 17 May 2020 Accepted: 13 July 2020 Published: 24 August 2020In addition to the recent emerged SARS-CoV-2, hepatitis B virus (HBV) is one of the viruses which cause a global infection and threat public health. In worldwide, the prevalence of HBsAg is about 3.9% (Polaris Observatory 2018). According to a nationwide epidemiological survey of population whose ages range from 1 to 59 years in China, 2006, the prevalence of HBsAg was 7.2% (Liang et al. 2009). As SARS-CoV-2 and HBV both can cause liver damage (Fan et al. 2020), further understanding of the risk of SARS-CoV-2 on patients with HBV infection is urgently required in order to design an optimized treatment strategy. However, the impacts of SARS-CoV-2 infection on HBV patients are still not clear. For example, we do not yet know whether the SARS-CoV-2 infection is more severe in HBV patients and we also do not have much knowledge about the impact of SARS-CoV-2 on the course of HBV infection. In this retrospective study, we investigated the clinical characterizes of the patients coinfected with SARS-CoV-2 and HBV by analyzing the clinical records and laboratory tests of 123 COVID-19 patients admitted to Zhongnan Hospital of Wuhan University, Wuhan, China, from January 5 to February 20, 2020. -
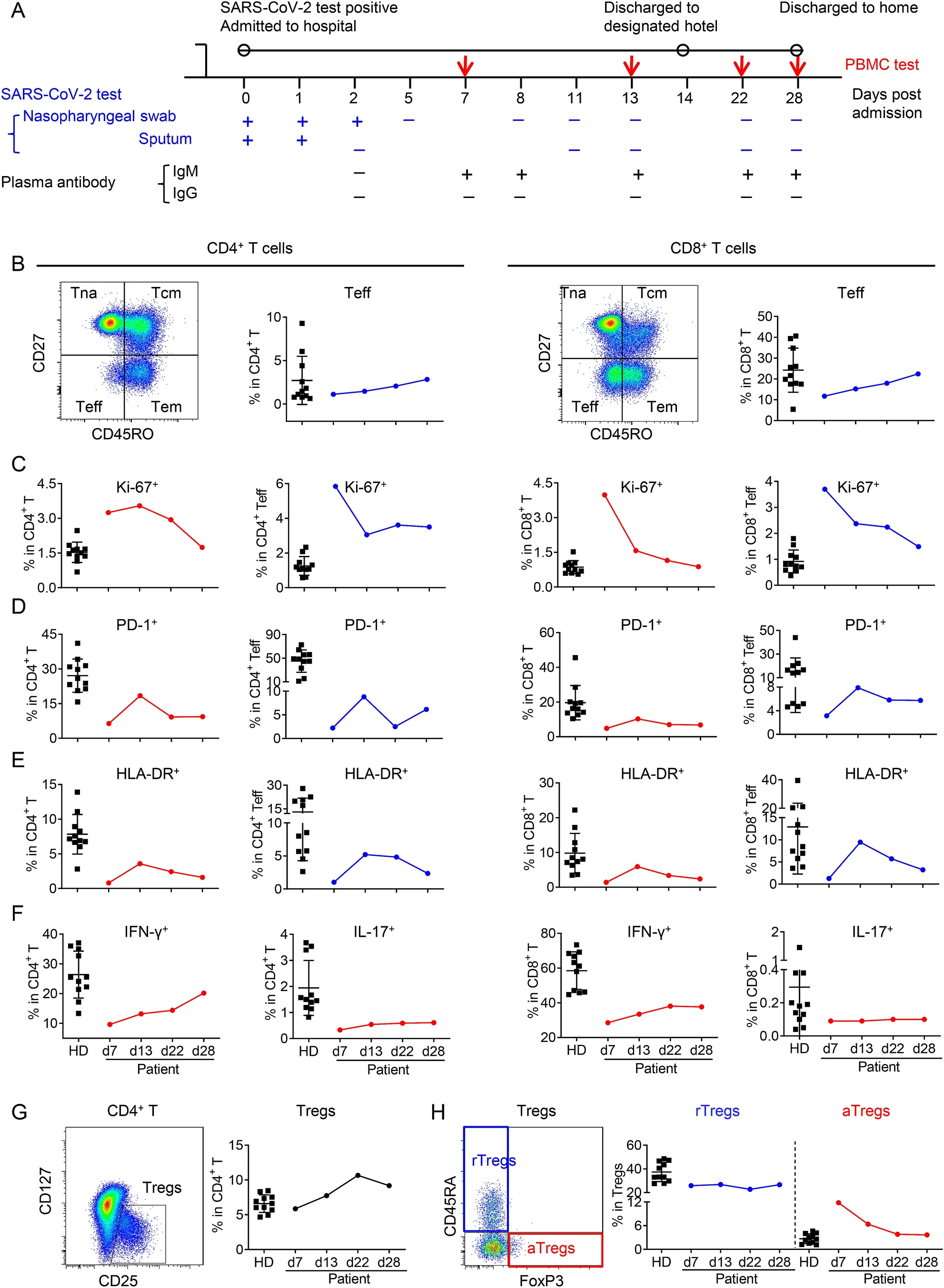
Longitudinal Characteristics of T Cell Responses in Asymptomatic SARS-CoV-2 Infection
2020, 35(6): 838 doi: 10.1007/s12250-020-00277-4
Received: 28 June 2020 Accepted: 24 July 2020 Published: 21 August 2020SARS-CoV-2 causes a spectrum of illness, ranging from an asymptomatic state to life-threatening multi-organ failure, and imposes a high socioeconomic burden on its sufferers and on society (Chen et al. 2020; Pan et al. 2020). Asymptomatic SARS-CoV-2 infection, confirmed by assay with quantitative real-time reverse transcription PCR (qRT-PCR), presented neither clinical symptom nor radiographic abnormality (Pan et al. 2020). While T cell responses are crucial for viral control, viral infection also induces significant count decrease and function impairment of T cells (Chen et al. 2020; Qin et al. 2020). Despite high rate of SARS-CoV-2 asymptomatic infections (Black et al. 2020), T cell responses of this population are still largely unknown. Here, we reported the kinetics of CD4+ and CD8+ T cell responses in an asymptomatic SARS-CoV-2 infected case. -
Patterns of Gustatory Recovery in Patients Affected by the COVID-19 Outbreak
2020, 35(6): 833 doi: 10.1007/s12250-020-00272-9
Received: 28 June 2020 Accepted: 15 July 2020 Published: 03 August 2020Coronavirus disease 2019 (COVID-19) is a viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). From March 2020, several studies indicate that many subjects affected by mild-to-moderate COVID-19 presented olfactory/gustatory dysfunction (OD/GD) that appeared strongly correlated between them but not with the other symptoms suggestive of upper airway infectionIn order to evaluate patterns of gustatoy recovery, data from patients with confirmed COVID-19 were collected prospectively from 4 University Hospitals. At this relatively early point in the pandemic, the authors considered that subjective patterns of recovery of olfactory disfunction in COVID-19 patients are valuable for our patients, for hypothesis generation and treatment development. -

Feasibility Study of Mixing Throat Swab Samples for Severe Acute Respiratory Syndrome Coronavirus-2 Screening
2020, 35(6): 830 doi: 10.1007/s12250-020-00254-x
Received: 12 May 2020 Accepted: 03 June 2020 Published: 08 July 2020At present, viral nucleic acids are generally sampled using throat swabs and detected by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). However, the capacity for SARS-CoV-2 nucleic acid detection is largely limited by the number of instruments, kits, and experienced laboratory personnel available. These limitations have caused low screening efficiency, which leads to a lag in identifying potential infections and may then exacerbate the spread of COVID-19 (Carter et al. 2020). Therefore, novel qRT-PCR approaches for nucleic acid detection are required to enhance testing efficiency.In this study, we aimed to explore a practical method and procedure for SARS-CoV-2 qRT-PCR detection in 96-well plates using pooled throat swab samples. This method may reduce the cost of testing and increase the screening capacity for SARS-CoV-2 using existing instrument and kits, consequently facilitating the containment of COVID-19 worldwide. -
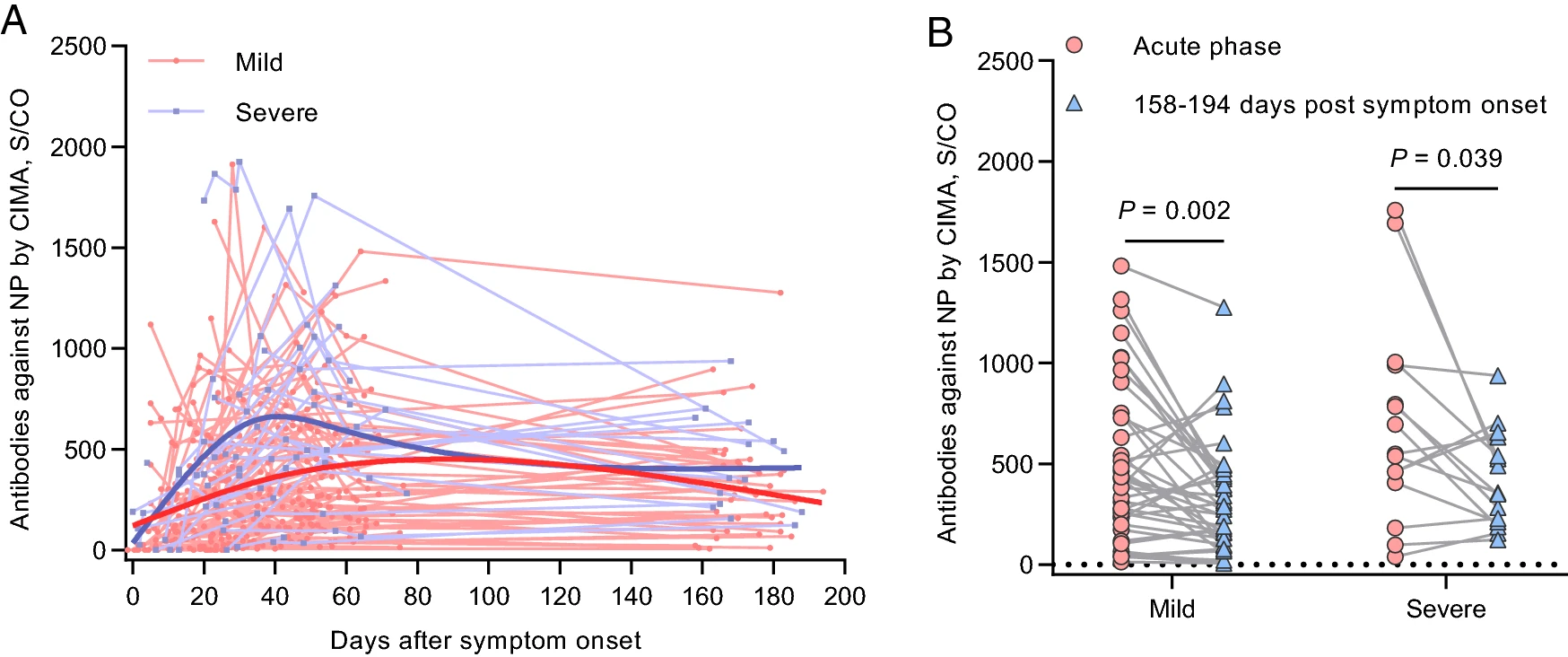
Viral and Antibody Kinetics of COVID-19 Patients with Different Disease Severities in Acute and Convalescent Phases: A 6-Month Follow-Up Study
2020, 35(6): 820 doi: 10.1007/s12250-020-00329-9
Received: 01 September 2020 Accepted: 23 November 2020 Published: 22 December 2020Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly around the world, posing a major threat to human health and the economy. Currently, long-term data on viral shedding and the serum antibody responses in COVID-19 patients are still limited. Herein, we report the clinical features, viral RNA loads, and serum antibody levels in a cohort of 112 COVID-19 patients admitted to the Honghu People's Hospital, Hubei Province, China. Overall, 5.36% (6/112) of patients showed persistent viral RNA shedding (> 45 days). The peak viral load was higher in the severe disease group than in the mild group (median cycle threshold value, 36.4 versus 31.5; P = 0.002). For most patients the disappearance of IgM antibodies occurred approximately 4-6 weeks after symptoms onset, while IgG persisted for over 194 days after the onset of symptoms, although patients showed a 46% reduction in antibodies titres against SARS-CoV-2 nucleocapsid protein compared with the acute phase. We also studied 18 asymptomatic individuals with RT-qPCR confirmed SARS-CoV-2 infection together with 17 symptomatic patients, and the asymptomatic individuals were the close contacts of these symptomatic cases. Delayed IgG seroconversion and lower IgM seropositive rates were observed in asymptomatic individuals. These data indicate that higher viral loads and stronger antibody responses are related to more severe disease status in patients with SARS-CoV-2 infection, and the antibodies persisted in the recovered patient for more than 6 months so that the vaccine may provide protection against SARS-CoV-2 infection. -
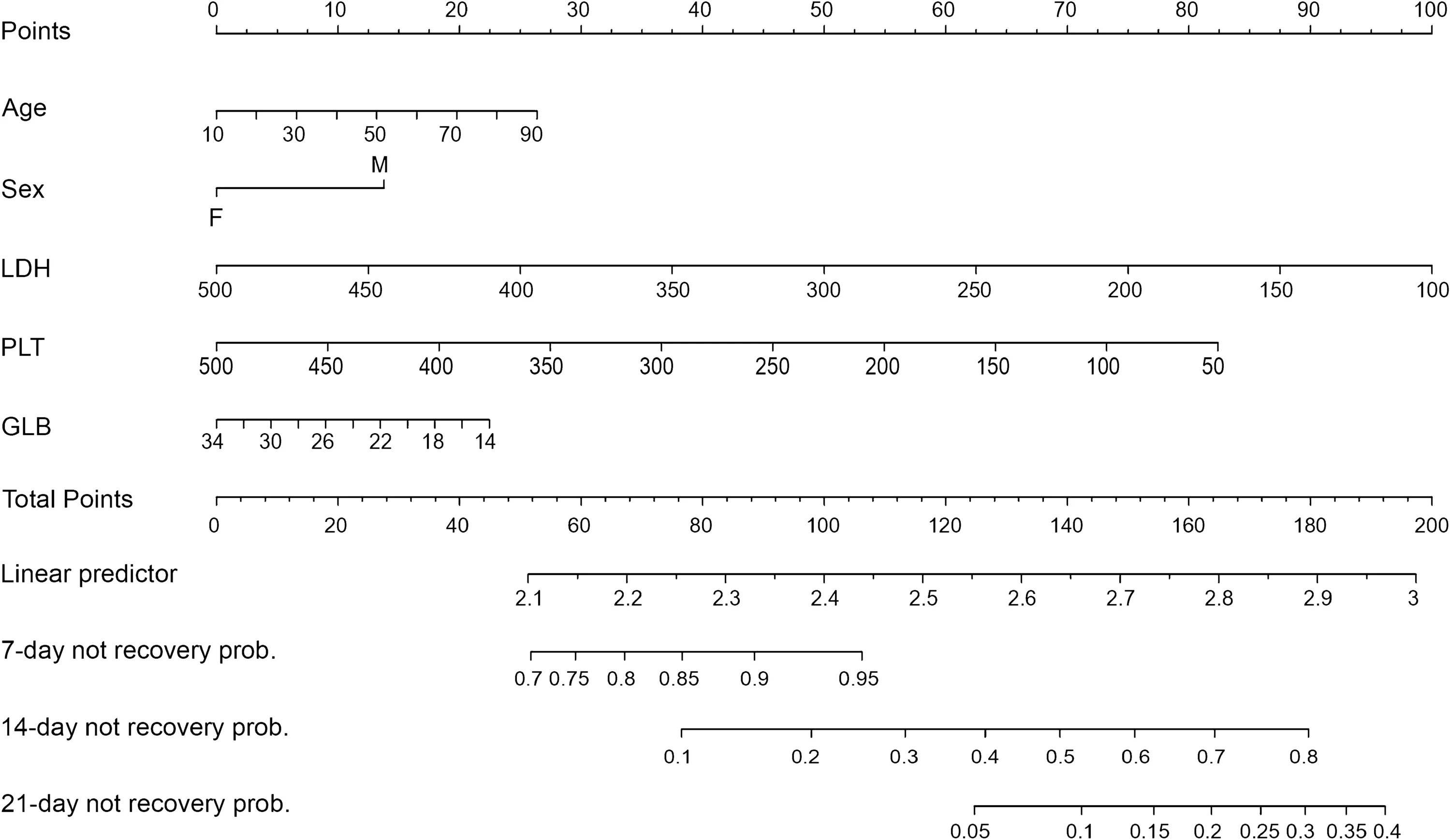
A Prognostic Model to Predict Recovery of COVID-19 Patients Based on Longitudinal Laboratory Findings
2020, 35(6): 811 doi: 10.1007/s12250-020-00317-z
Received: 01 June 2020 Accepted: 09 October 2020 Published: 10 November 2020The temporal change patterns of laboratory data may provide insightful clues into the whole course of COVID-19. This study aimed to evaluate longitudinal change patterns of key laboratory tests in patients with COVID-19, and identify independent prognostic factors by examining the associations between laboratory findings and outcomes of patients. This multicenter study included 56 patients with COVID-19 treated in Jilin Province, China, from January 21, 2020 to March 5, 2020. The laboratory findings, epidemiological characteristics and demographic data were extracted from electronic medical records. The average value of eosinophils and carbon dioxide combining power continued to significantly increase, while the average value of cardiac troponin I and mean platelet volume decreased throughout the course of the disease. The average value of lymphocytes approached the lower limit of the reference interval for the first 5 days and then rose slowly thereafter. The average value of thrombocytocrit peaked on day 7 and slowly declined thereafter. The average value of mean corpuscular volume and serum sodium showed an upward trend from day 8 and day 15, respectively. Age, sex, lactate dehydrogenase, platelet count and globulin level were included in the final model to predict the probability of recovery. The above parameters were verified in 24 patients with COVID-19 in another area of Jilin Province. The risk stratification and management of patients with COVID-19 could be improved according to the temporal trajectories of laboratory tests. -
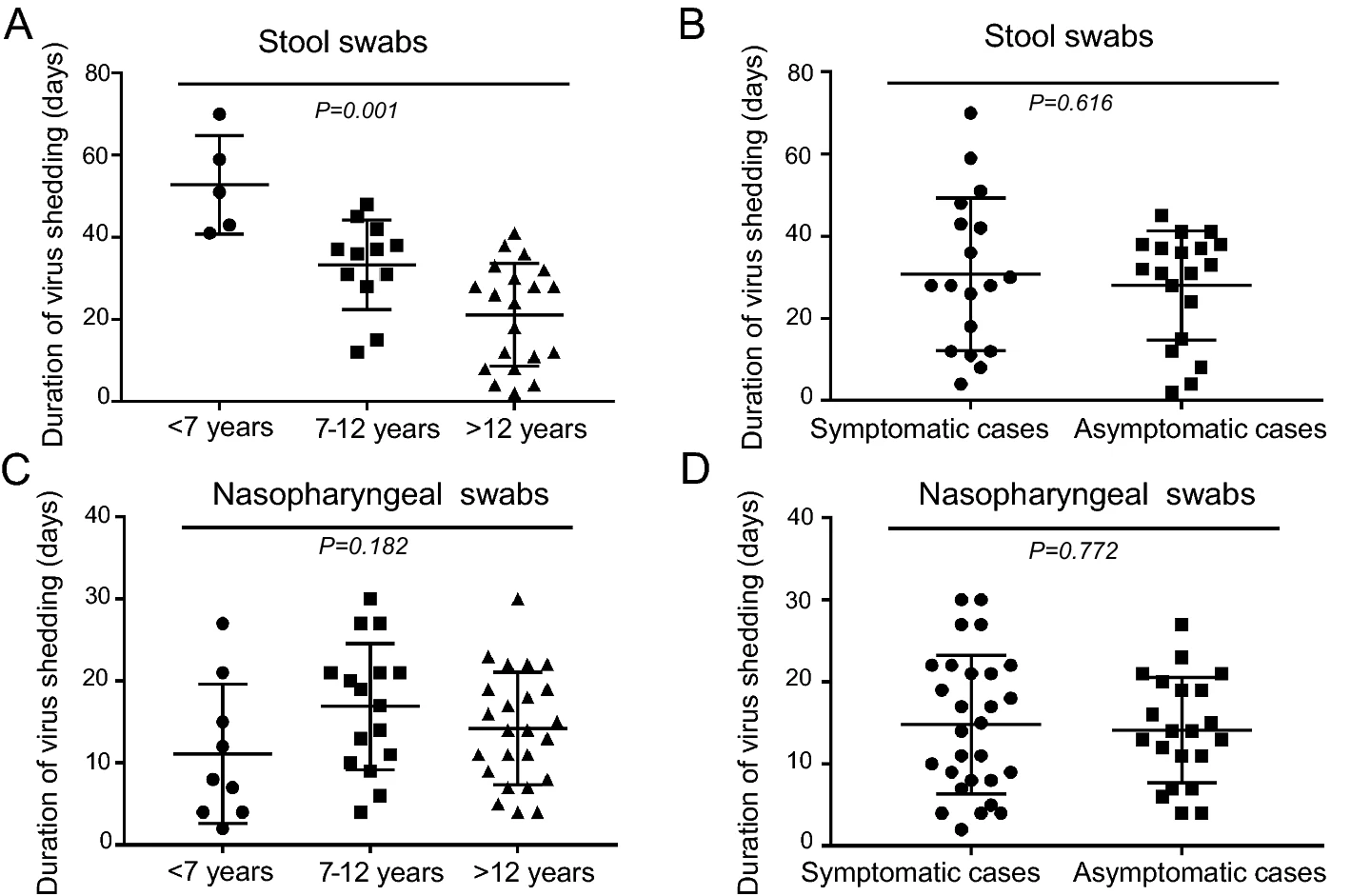
Comparison of Clinical and Epidemiological Characteristics of Asymptomatic and Symptomatic SARS-CoV-2 Infection in Children
2020, 35(6): 803 doi: 10.1007/s12250-020-00312-4
Received: 19 July 2020 Accepted: 11 October 2020 Published: 04 November 2020To understand the epidemiological and clinical features of the symptomatic and asymptomatic pediatric cases of COVID-19, we carried out a prospective study in Shanghai during the period of January 19 to April 30, 2020. A total of 49 children (mean age 11.5 ± 5.12 years) confirmed with SARS-CoV-2 infection were enrolled in the study, including 11 (22.4%) domestic cases and 38 (77.6%) imported cases. Nine (81.8%) local cases and 12 (31.6%) imported cases had a definitive epidemiological exposure. Twenty-eight (57.1%) were symptomatic and 21 (42.9%) were asymptomatic. Neither asymptomatic nor symptomatic cases progressed to severe diseases. The mean duration of viral shedding for SARS-CoV-2 in upper respiratory tract was 14.1 ± 6.4 days in asymptomatic cases and 14.8 ± 8.4 days in symptomatic cases (P > 0.05). Forty-five (91.8%) cases had viral RNA detected in stool. The mean duration of viral shedding in stool was 28.1 ± 13.3 days in asymptomatic cases and 30.8 ± 18.6 days in symptomatic participants (P > 0.05). Children < 7 years shed viral RNA in stool for a longer duration than school-aged children (P < 0.05). Forty-three (87.8%) cases had seropositivity for antibodies against SARS-CoV-2 within 1–3 weeks after confirmation with infection. In conclusion, asymptomatic SARS-CoV-2 infection may be common in children in the community during the COVID-19 pandemic wave. Asymptomatic cases shed viral RNA in a similar pattern as symptomatic cases do. It is of particular concern that asymptomatic individuals are potentially seed transmission of SARS-CoV-2 and pose a challenge to disease control. -
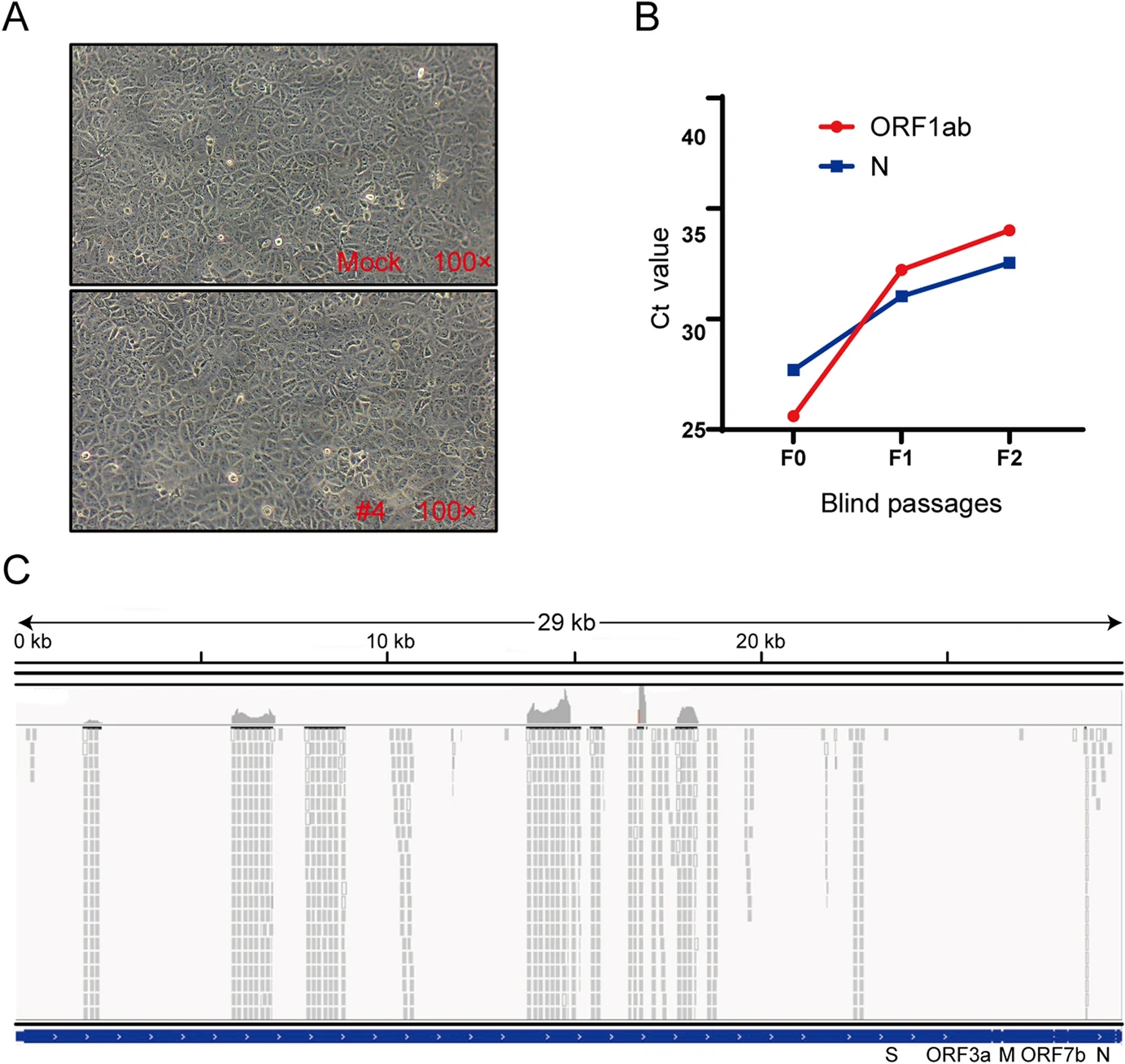
Long-Term Existence of SARS-CoV-2 in COVID-19 Patients: Host Immunity, Viral Virulence, and Transmissibility
2020, 35(6): 793 doi: 10.1007/s12250-020-00308-0
Received: 13 August 2020 Accepted: 10 September 2020 Published: 06 November 2020COVID-19 patients can recover with a median SARS-CoV-2 clearance of 20 days post initial symptoms (PIS). However, we observed some COVID-19 patients with existing SARS-CoV-2 for more than 50 days PIS. This study aimed to investigate the cause of viral clearance delay and the infectivity in these patients. Demographic data and clinical characteristics of 22 long-term COVID-19 patients were collected. The median age of the studied cohort was 59.83 ± 12.94 years. All patients were clinically cured after long-term SARS-CoV-2 infection ranging from 53 to 112 days PIS. Peripheral lymphocytes counts were normal. The ratios of interferon gamma (IFN-γ)-secreting cells to total CD4+ and CD8+ cells were normal as 24.68% ± 9.60% and 66.41% ± 14.87% respectively. However, the number of IFN-γ-secreting NK cells diminished (58.03% ± 11.78%). All patients presented detectable IgG, which positively correlated with mild neutralizing activity (Mean value neutralisation antibodies titers = 157.2, P= 0.05). No SARS-CoV-2 virus was isolated in Vero E6 cells inoculated with nasopharyngeal swab samples from all patients 50 days PIS, and the cytopathic effect was lacking. But one sample was positive for SARS-CoV-2 nucleic acid test in cell supernatants after two passages. Genome sequencing revealed that only three synonymous variants were identified in spike protein coding regions. In conclusion, decreased IFN-γ production by NK cells and low neutralizing antibodies might favor SARS-CoV-2 long-term existence. Further, low viral load and weak viral pathogenicity were observed in COVID-19 patients with long-term SARS-CoV-2 infection. -
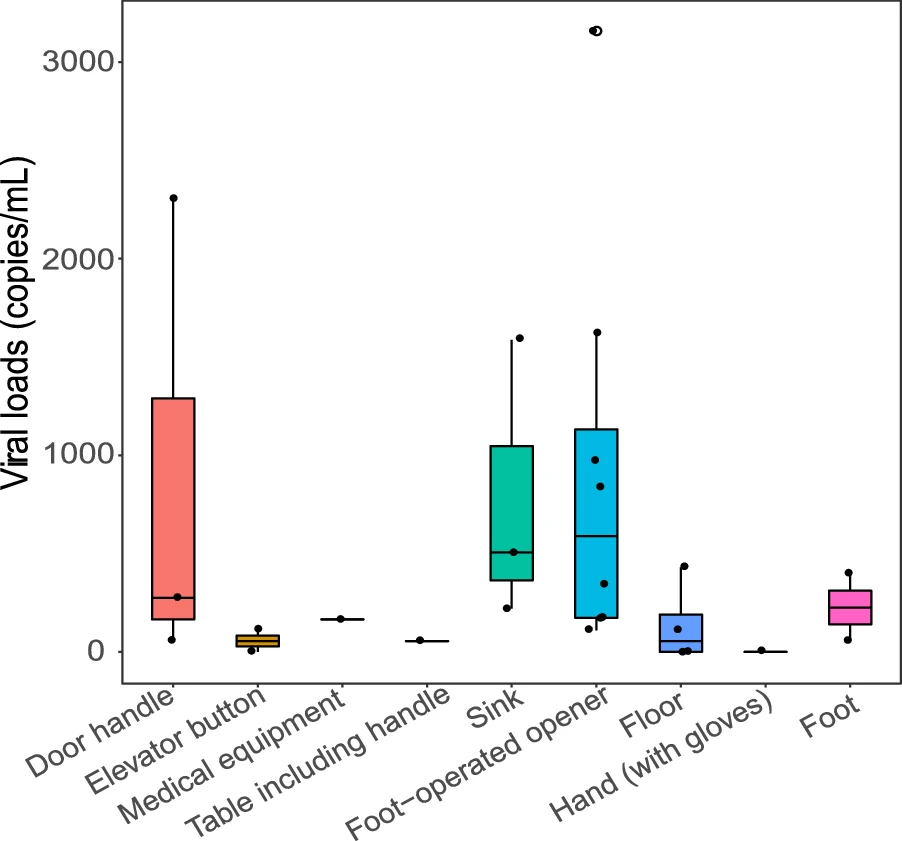
Identifying the Risk of SARS-CoV-2 Infection and Environmental Monitoring in Airborne Infectious Isolation Rooms (AIIRs)
2020, 35(6): 785 doi: 10.1007/s12250-020-00301-7
Received: 28 May 2020 Accepted: 31 August 2020 Published: 28 September 2020Healthcare workers (HCWs) are at high risk of occupational exposure to the new pandemic human coronavirus, SARS-CoV-2, and are a source of nosocomial transmission in airborne infectious isolation rooms (AIIRs). Here, we performed comprehensive environmental contamination surveillance to evaluate the risk of viral transmission in AIIRs with 115 rooms in three buildings at the Shanghai Public Health Clinical Center, Shanghai, during the treatment of 334 patients infected with SARS-CoV-2. The results showed that the risk of airborne transmission of SARS-CoV-2 in AIIRs was low (1.62%, 25/1544) due to the directional airflow and strong environmental hygiene procedures. However, we detected viral RNA on the surface of foot-operated openers and bathroom sinks in AIIRs (viral load: 55.00–3154.50 copies/mL). This might be a source of contamination to connecting corridors and object surfaces through the footwear and gloves used by HCWs. The risk of infection was eliminated by the use of disposable footwear covers and the application of more effective environmental and personal hygiene measures. With the help of effective infection control procedures, none of 290 HCWs was infected when working in the AIIRs at this hospital. This study has provided information pertinent for infection control in AIIRs during the treatment of COVID-19 patients. -
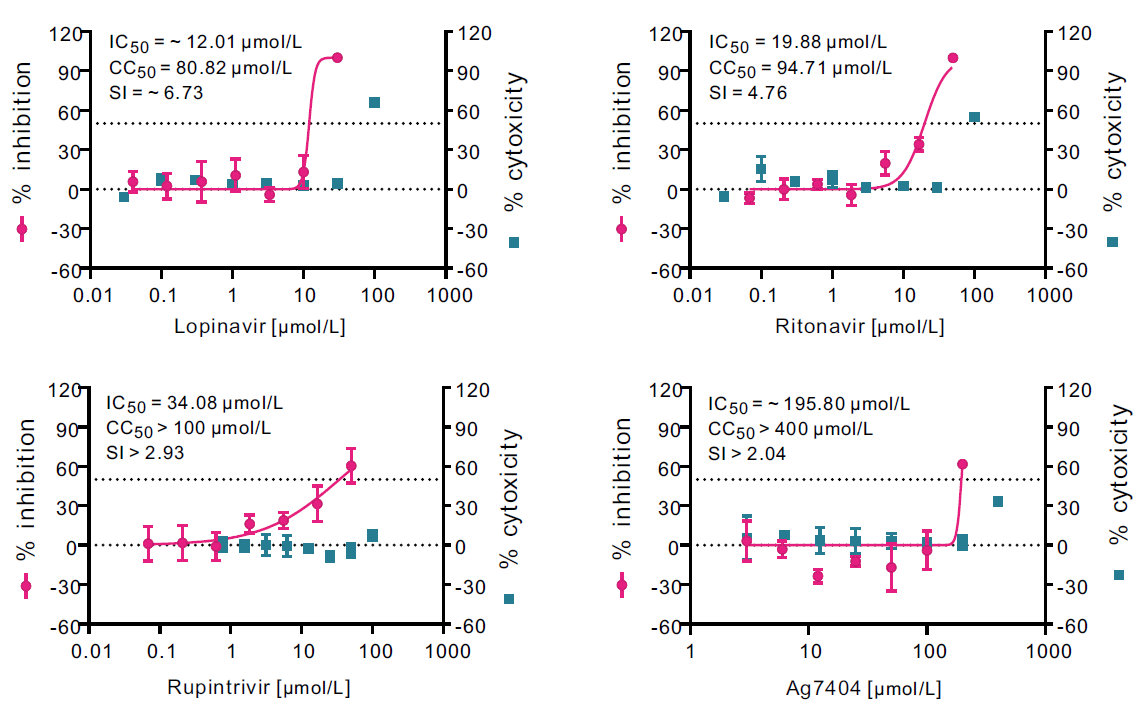
Comparative Antiviral Efficacy of Viral Protease Inhibitors against the Novel SARS-CoV-2 In Vitro
2020, 35(6): 776 doi: 10.1007/s12250-020-00288-1
Received: 04 August 2020 Accepted: 14 August 2020 Published: 10 September 2020The recent outbreak of novel coronavirus pneumonia (COVID-19) caused by a new coronavirus has posed a great threat to public health. Identifying safe and effective antivirals is of urgent demand to cure the huge number of patients. Virus-encoded proteases are considered potential drug targets. The human immunodeficiency virus protease inhibitors (lopinavir/ritonavir) has been recommended in the global Solidarity Trial in March launched by World Health Organization. However, there is currently no experimental evidence to support or against its clinical use. We evaluated the antiviral efficacy of lopinavir/ritonavir along with other two viral protease inhibitors in vitro, and discussed the possible inhibitory mechanism in silico. The in vitro to in vivo extrapolation was carried out to assess whether lopinavir/ritonavir could be effective in clinical. Among the four tested compounds, lopinavir showed the best inhibitory effect against the novel coronavirus infection. However, further in vitro to in vivo extrapolation of pharmacokinetics suggested that lopinavir/ritonavir could not reach effective concentration under standard dosing regimen [marketed as Kaletra®, contained lopinavir/ritonavir (200 mg/50 mg) tablets, recommended dosage is 400 mg/10 mg (2 tablets) twice daily]. This research concluded that lopinavir/ritonavir should be stopped for clinical use due to the huge gap between in vitro IC50 and free plasma concentration. Nevertheless, the structure–activity relationship analysis of the four inhibitors provided further information for de novel design of future viral protease inhibitors of SARS-CoV-2. -
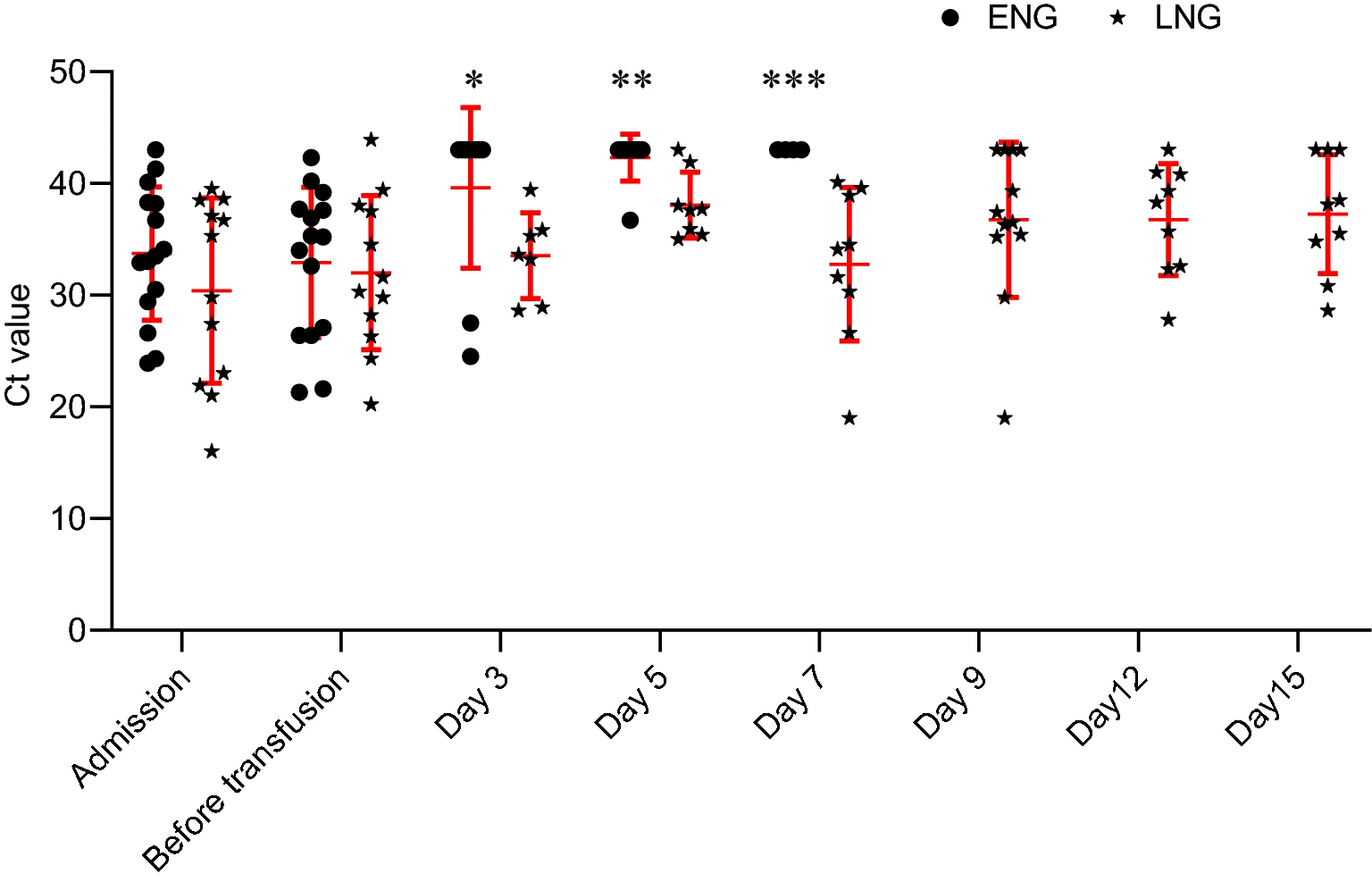
Patients with Prolonged Positivity of SARS-CoV-2 RNA Benefit from Convalescent Plasma Therapy: A Retrospective Study
2020, 35(6): 768 doi: 10.1007/s12250-020-00281-8
Received: 09 July 2020 Accepted: 03 August 2020 Published: 31 August 2020Convalescent plasma therapy has been implemented in a few cases of severe coronavirus disease 2019. No report about convalescent plasma therapy in treating patients with prolonged positivity of SARS-CoV-2 RNA has been published. In this study, we conducted a retrospective observational study in 27 patients with prolonged positivity of SARS-CoV-2 RNA, the clinical benefit of convalescent plasma therapy were analyzed. qRT-PCR test of SARS-CoV-2 RNA turned negative (≤ 7 days) in a part of patients (early negative group, n = 15) after therapy, others (late negative group, n = 12) turned negative in more than 7 days. Pulmonary imaging improvement was confirmed in 7 patients in early negative group and 8 in late negative group after CP therapy. Viral load decreased in early negative group compared with late negative group at day 3, 5, 7 after implementing convalescent plasma therapy. Patients in early negative group had a shorter median length of hospital stay. In conclusion, convalescent plasma therapy might help eliminate virus and shorten length of hospital stay in patients with prolonged positivity of SARS-CoV-2 RNA.
- First
- Prev
- 1
- 2
- Next
- Last
- Total:2
- To
- Go