2021 Vol.36(3)
Human adenoviruses (HAdVs) are non-enveloped double-stranded DNA viruses which belong to the genus Mastadenovirus. To date, over 100 genotypes of HAdVs have been recognized and are classified into seven species (A–G). HAdVs are highly contagious that could cause multiple systemic infections, such as acute respiratory disease, epidemic keratoconjunctivitis, hemorrhagic cystitis, gastroenteritis, as well as other diseases. HAdVs-associated respiratory tract infection is one of the leading causes of severe respiratory tract diseases in pediatric populations, characterized by poor prognosis, high mortality, severe complications, and serious sequelae. However, there is no recommended effective drug for adenovirus infections. To further advance our knowledge on HAdVs, we dedicate a special topic covering the latest researches on clinical diagnosis, epidemiology, detection methods, vaccine development, and HAdV-associated severe diseases. The cover is modified from an electron micrograph of the rAd3H recombinant vaccine virus (kindly provided by Prof. Qiwei Zhang) with artistic processing. See page 354–364 for details.
|
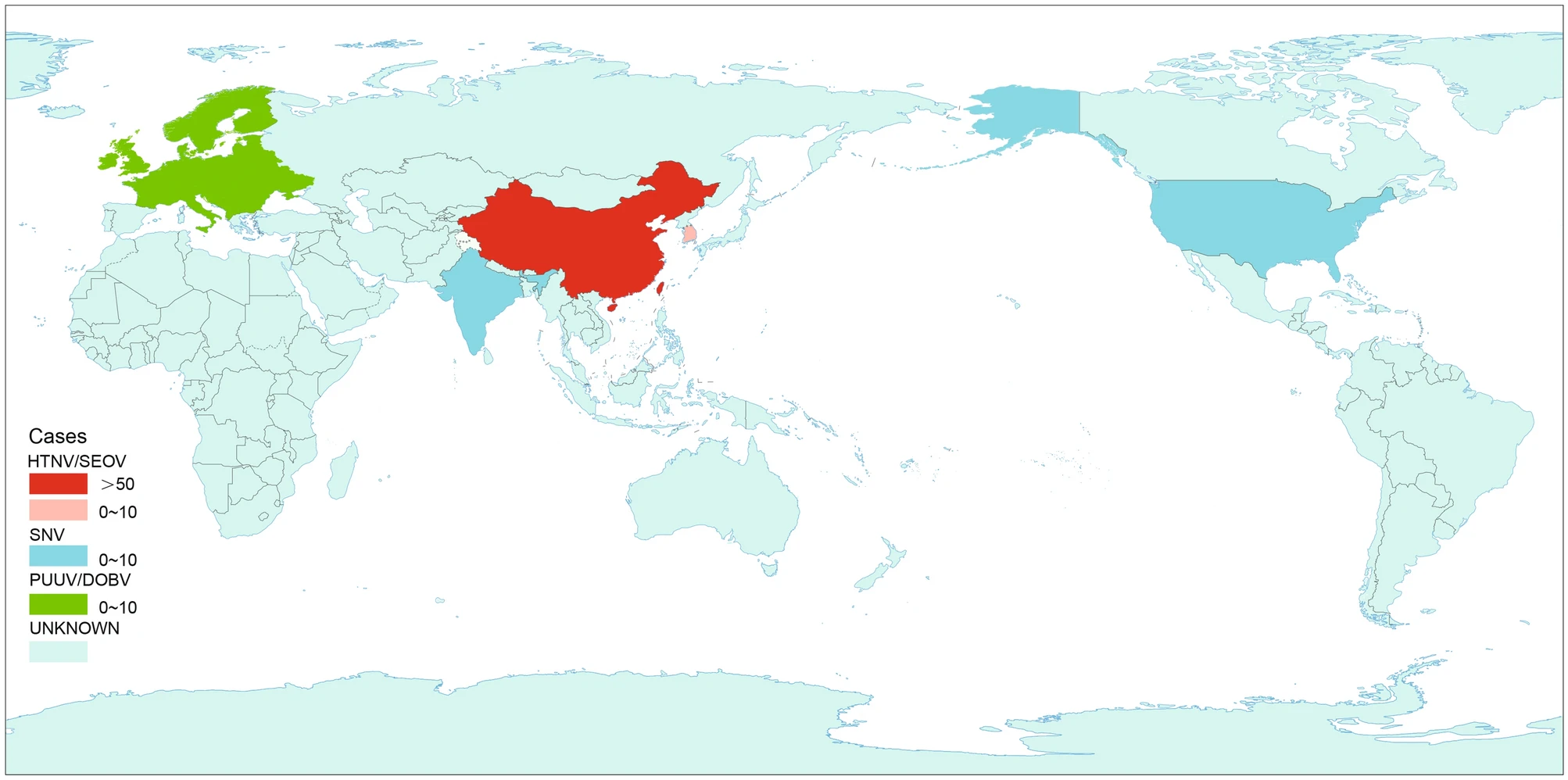
Hantavirus Infection during Pregnancy
2021, 36(3): 345 doi: 10.1007/s12250-020-00300-8
Hantavirus infection is a global health challenge, causing widespread public concern. In recent years, cases of hantavirus infection in pregnant women have been reported in many countries. The infected pregnant women and their fetuses appear to have more severe clinical symptoms and worse clinical outcomes. Hence, to study the prevalence of hantavirus infection in pregnant women, this study will focus on the epidemiological distribution of the virus, different virus species penetrating the placental barrier, and factors affecting the incidence and clinical outcome of the infection in pregnant women and their fetuses. In addition, this review will also discuss the diagnostic tools and treatments for pregnant patients and provide an overview of the relevant future research.
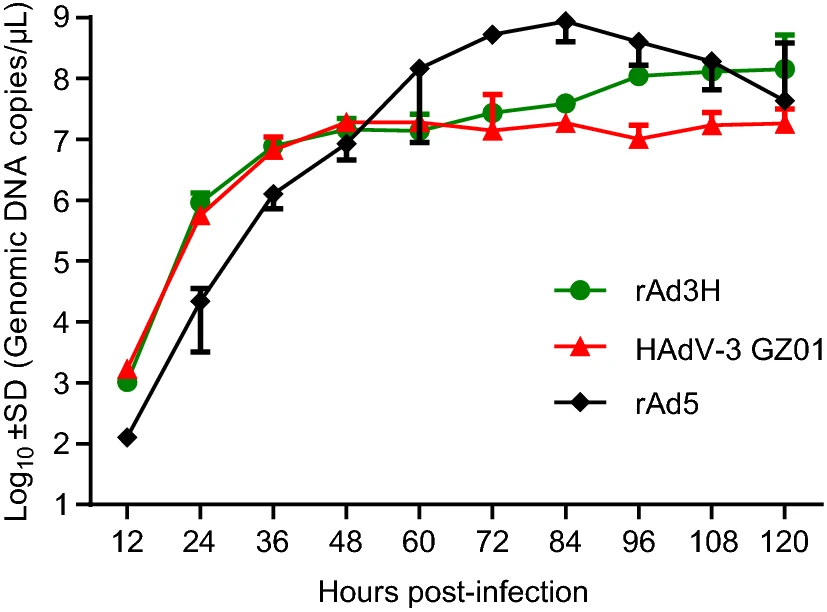
Construction and Characterization of a Novel Recombinant Attenuated and Replication-Deficient Candidate Human Adenovirus Type 3 Vaccine: "Adenovirus Vaccine Within an Adenovirus Vector"
2021, 36(3): 354 doi: 10.1007/s12250-020-00234-1
Human adenoviruses (HAdVs) are highly contagious and result in large number of acute respiratory disease (ARD) cases with severe morbidity and mortality. Human adenovirus type 3 (HAdV-3) is the most common type that causes ARD outbreaks in Asia, Europe, and the Americas. However, there is currently no vaccine approved for its general use. The hexon protein contains the main neutralizing epitopes, provoking strong and lasting immunogenicity. In this study, a novel recombinant and attenuated adenovirus vaccine candidate against HAdV-3 was constructed based on a commercially-available replication-defective HAdV-5 gene therapy and vaccine vector. The entire HAdV-3 hexon gene was integrated into the E1 region of the vector by homologous recombination using a bacterial system. The resultant recombinants expressing the HAdV-3 hexon protein were rescued in AD293 cells, identified and characterized by RT-PCR, Western blots, indirect immunofluorescence, and electron microscopy. This potential vaccine candidate had a similar replicative efficacy as the wild-type HAdV-3 strain. However, and importantly, the vaccine strain had been rendered replication-defective and was incapable of replication in A549 cells after more than twenty-generation passages in AD293 cells. This represents a significant safety feature. The mice immunized both intranasally and intramuscularly by this vaccine candidate raised significant neutralizing antibodies against HAdV-3. Therefore, this recombinant, attenuated, and safe adenovirus vaccine is a promising HAdV-3 vaccine candidate. The strategy of using a clinically approved and replication-defective HAdV-5 vector provides a novel approach to develop universal adenovirus vaccine candidates against all the other types of adenoviruses causing ARDs and perhaps other adenovirus-associated diseases.
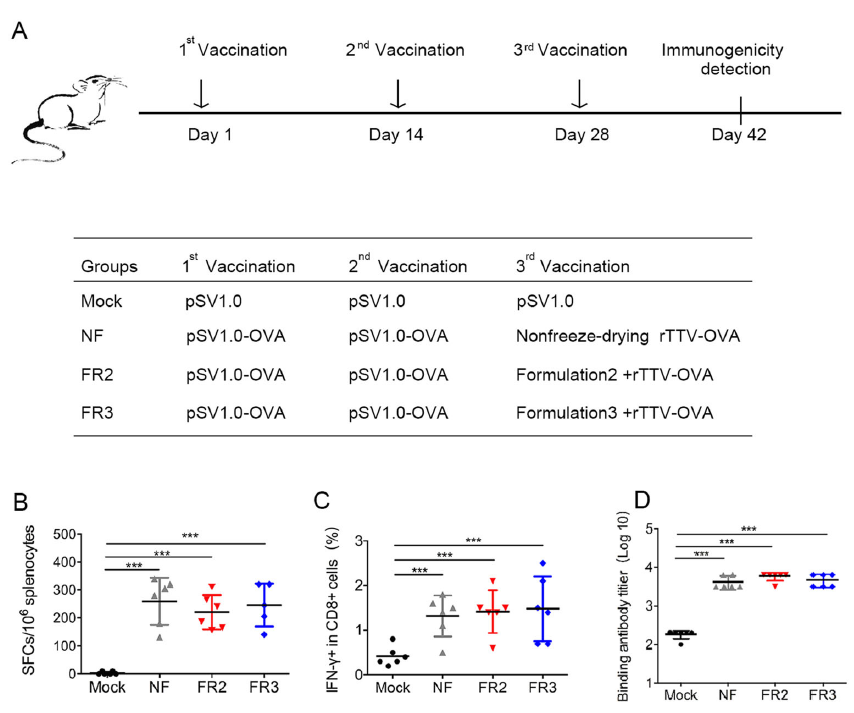
Freeze-Drying Formulations Increased the Adenovirus and Poxvirus Vaccine Storage Times and Antigen Stabilities
2021, 36(3): 365 doi: 10.1007/s12250-020-00250-1
Successful vaccines induce specific immune responses and protect against various viral and bacterial infections. Noninactivated vaccines, especially viral vector vaccines such as adenovirus and poxvirus vaccines, dominate the vaccine market because their viral particles are able to replicate and proliferate in vivo and produce lasting immunity in a manner similar to natural infection. One challenge of human and livestock vaccination is vaccine stability related to the antigenicity and infectivity. Freeze-drying is the typical method to maintain virus vaccine stability, while cold chain transportation is required for temperatures about 2 ℃–8 ℃. The financial and technological resource requirements hinder vaccine distribution in underdeveloped areas. In this study, we developed a freeze-drying formula consisting of bovine serum albumin (BSA), L-glutamic acid (L-Glu), polyethylene glycol (PEG), and dextran (DEX) to improve the thermal stability and activity of viral vaccines, including vaccinia recombinant vaccine (rTTV-OVA) and adenovirus vaccine (Ad5-ENV). We compared a panel of five different formulations (PEG: DEX: BSA: L-GLU=50:9:0:0(#1), 50:5:4:0(#2), 50:10:9:0(#3), 50:0:0:9(#4), and 50:1:0:8(#5), respectively) and optimized the freeze-drying formula for rTTV-OVA and Ad5-ENV. We found that the freeze-drying formulations #2 and #3 could maintain rTTV-OVA infectivity at temperatures of 4 ℃ and 25 ℃ and that rTTV-OVA immunogenicity was retained during lyophilization. However, formulations #4 and #5 maintained Ad5-ENV infectivity under the same conditions, and Ad5-ENV immunogenicity had maximum retention with freeze-drying formulation #4. In summary, we developed new freeze-drying formulations that increased virus vaccine storage times and retained immunogenicity at an ambient temperature.
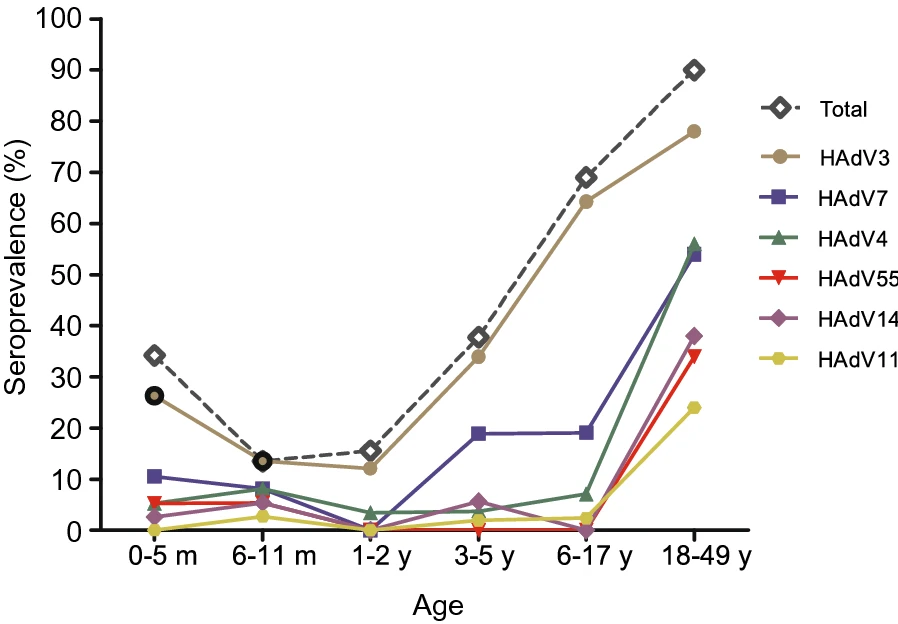
Seroprevalence of Neutralizing Antibodies against Six Human Adenovirus Types Indicates the Low Level of Herd Immunity in Young Children from Guangzhou, China
2021, 36(3): 373 doi: 10.1007/s12250-020-00307-1
Human adenoviruses (HAdVs) commonly cause many diseases such as respiratory diseases, gastroenteritis, cystitis worldwide. HAdV-3, -7, -4 and emergent HAdV-55 and HAdV-14 are the most important types causing severe respiratory diseases. There is no effective drug available for clinical treatment, and no vaccine available for the general population. Therefore, it is important to investigate the seroprevalence against HAdV for developing novel vaccines and vectors. In this study, we investigated the seroprevalence and titer levels of neutralizing antibodies (NAb) against HAdV-3, -4, -7, -14, -55, and -11 in total 278 healthy populations between 0 months and 49 years of age (228 children and 50 adults) from Guangzhou. In children under the age of 18 years, the seropositive rates were significantly increased against HAdV-3 at 12.07%, 33.96%, and 64.29% and against HAdV-7 at 0%, 18.87%, and 19.05% in age groups of 1–2, 3–5, and 6–17 years, respectively. The seroprevalence was very low (0%~8.1%) for all other four types. In adults aged between 18 and 49 years, HAdV-3, -4, and -7 (>50.00%) were the most common types, followed by HAdV-14 (38.00%), -55 (34.00%), and -11 (24.00%). Adults tended to have high NAb titers against HAdV-4 and -55. HAdV-55-seropositive donors tended to be HAdV-11- and HAdV-14-seropositive. These results indicated the low level of herd immunity against all six HAdV types in young children, and HAdV-14, -55, -11 in adults from Guangzhou City. Our findings demonstrate the importance of monitoring HAdV types and developing vaccines against HAdV for children and adults.
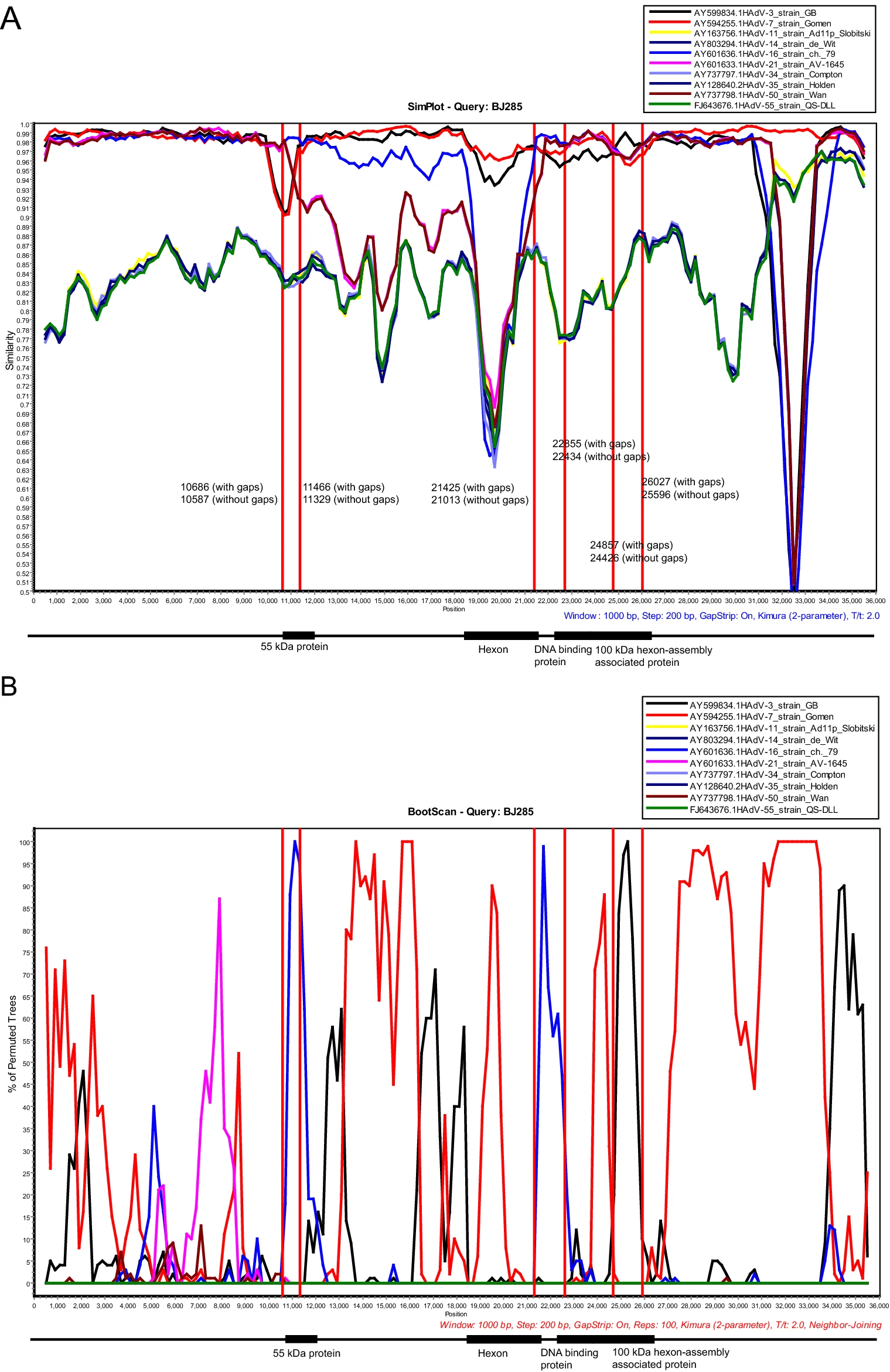
Genetic Analysis of Human Adenovirus Type 7 Strains Circulating in Different Parts of China
2021, 36(3): 382 doi: 10.1007/s12250-020-00334-y
To investigate the molecular epidemiology and genetic variation of human adenovirus type 7 (HAdV-7) in children with acute respiratory infections (ARI) in China. HAdV-7-positive respiratory samples collected from children with ARI in Beijing, Shijiazhuang, Wenzhou and Guangzhou from 2014–2018 were selected for gene amplification and sequence analysis. Fifty-seven HAdV-7 clinical strains with hexon, penton base and fiber gene sequences were obtained. Meanwhile 17 strains were selected randomly from different cities for whole genome sequencing. Phylogenetic and variation analyses were performed based on the obtained sequences, HAdV-7 prototype strain Gomen (AY594255), vaccine strains (AY495969 and AY594256) and representative sequences of strains. The phylogenetic trees constructed based on whole genome sequences, major capsid protein genes (hexon, penton base and fiber) and the early genes (E1, E2, E3 and E4) were not completely consistent. The HAdV-7 strains obtained in this study always clustered with most of the circulating strains worldwide from the 1980s to the present. Compared with the HAdV-7 prototype strain Gomen (AY594255), some amino acid mutations in loop1 and loop2 of hexon and the RGD loop region of the penton base gene were observed. Recombination analysis showed that partial regions of 55 kDa protein and 100 kDa hexon-assembly associated protein genes among all HAdV-7 strains in this study were from HAdV-16 and HAdV-3, respectively. Our study demonstrated the molecular evolution characteristics of HAdV-7 strains circulating in China and provided basic reference data for the prevention, control and vaccine development of HAdV-7.
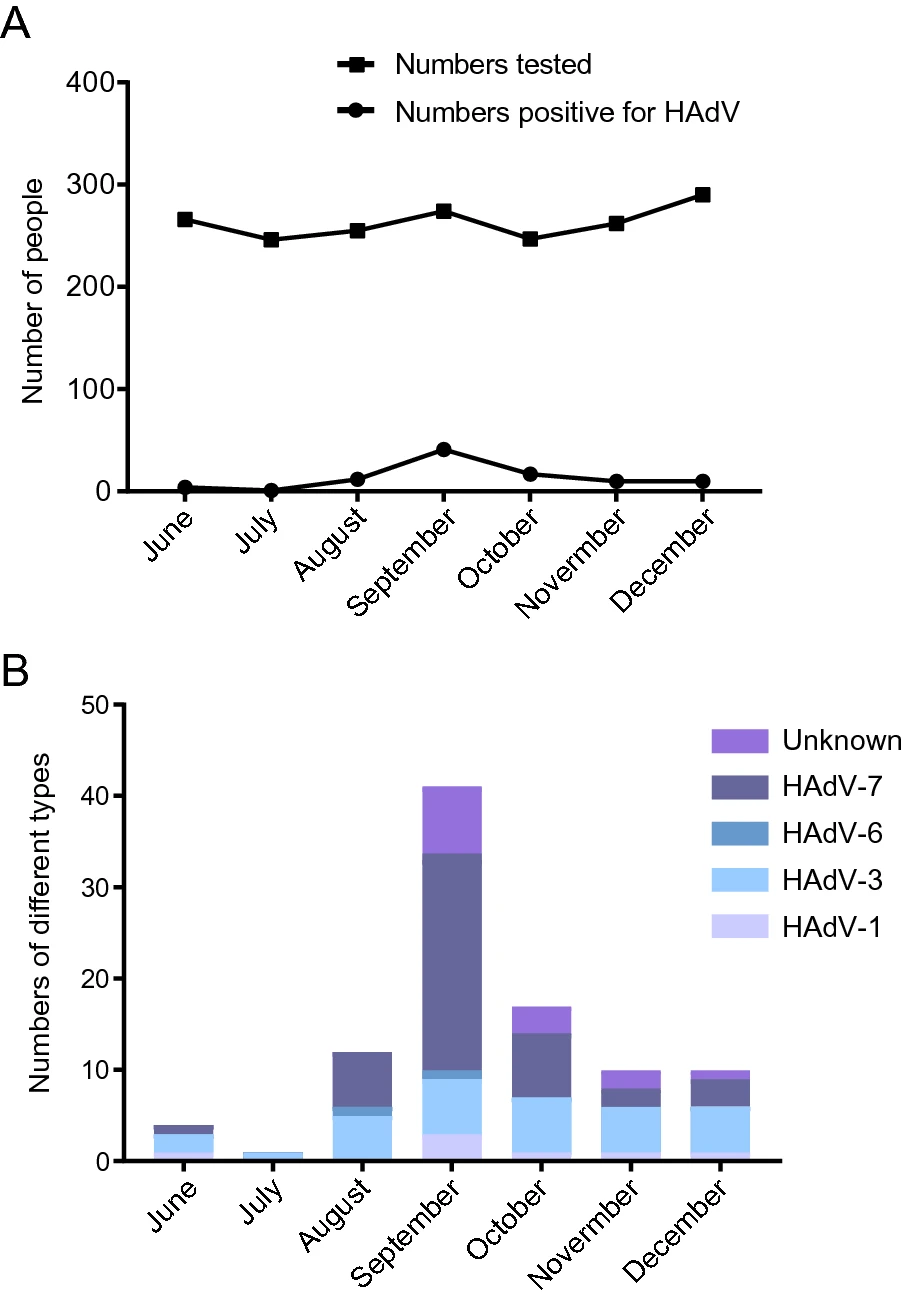
Application of Human Adenovirus Genotyping by Phylogenetic Analysis in an Outbreak to Identify Nosocomial Infection
2021, 36(3): 393 doi: 10.1007/s12250-020-00299-y
Nosocomial infections are common in pediatric patients and can be fatal in infants and immunocompromised patients. In September 2018, a high positive rate of human adenovirus HAdV was occurred among hospitalized children in the Children's Hospital Affiliated to the Capital Institute of Paediatrics in Beijing. To investigate whether this outbreak of HAdV was related to nosocomial infections or the result of community infections, we collected respiratory specimens from patients with acute respiratory infections in a respiratory ward during June to December 2018, and screened for respiratory viruses. Among 1, 840 cases included, 95 (5.2%, 95/1840) were positive for HAdV and 81 were genotyped based on phylogenetic analysis, including seven as HAdV-1 (8.6%), 30 HAdV-3 (37.0%), two HAdV-6 (2.5%), and 42 HAdV-7 (51.9%). More HAdV-positive samples were collected in August (4.7%, 12/255), September (15.0%, 41/274) and October (6.9%, 17/247), with a peak in September 2018. By combining the results of HAdV phylogenetic analysis with clinical data of patients, there were 77 cases (4.2%, 77/1840; 81.1%, 77/95) excluded from nosocomial infections, eight cases representing possible infections transmitted by visitors or attending parents, three cases without sequences that might have been due to infection transmitted by roommates positive for HAdV, one case of a roommate without an HAdV sequence, and six cases that shared highly homologous sequences with those of their roommates, for which nosocomial infections might be considered. In conclusion, genotyping of HAdVs based on phylogenetic analysis combined with clinical information provides a powerful method to distinguish nosocomial infections from community acquired infection, especially when tracing the origins of nosocomial infections.
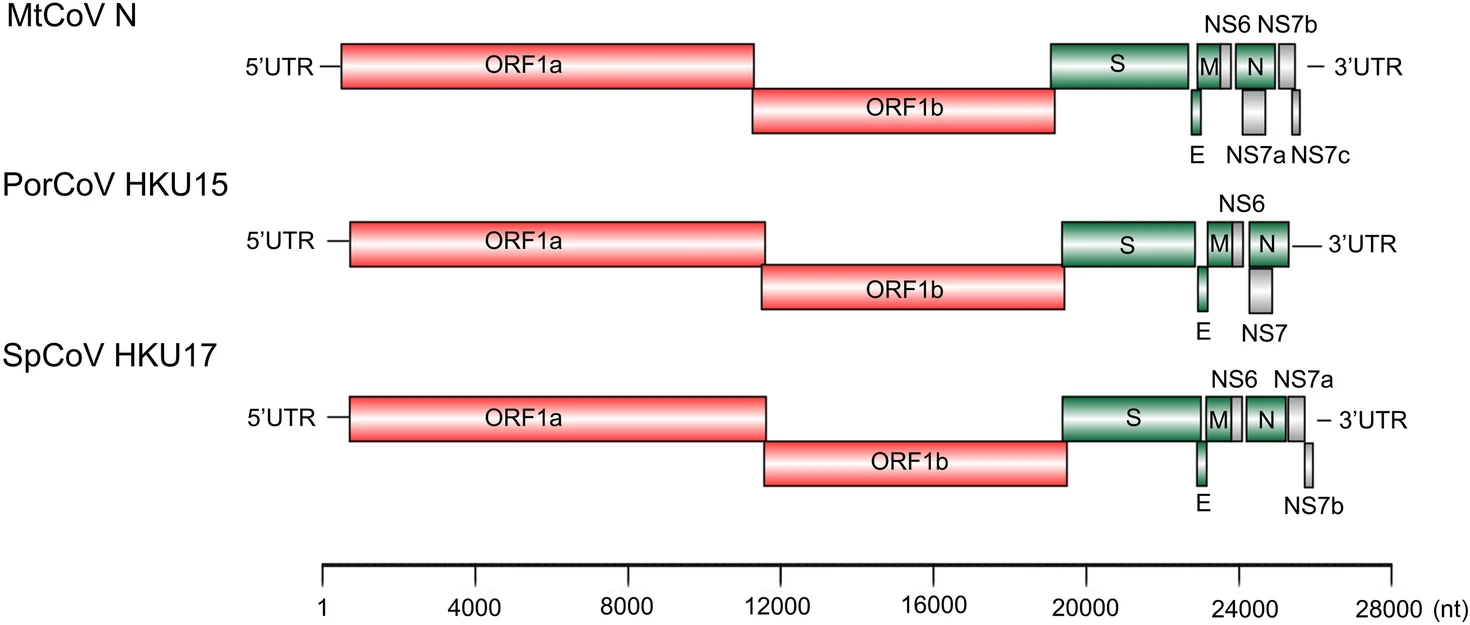
Beta- and Novel Delta-Coronaviruses Are Identified from Wild Animals in the Qinghai-Tibetan Plateau, China
2021, 36(3): 402 doi: 10.1007/s12250-020-00325-z
Outbreaks of severe virus infections with the potential to cause global pandemics are increasingly concerning. One type of those commonly emerging and re-emerging pathogens are coronaviruses (SARS-CoV, MERS-CoV and SARS-CoV-2). Wild animals are hosts of different coronaviruses with the potential risk of cross-species transmission. However, little is known about the reservoir and host of coronaviruses in wild animals in Qinghai Province, where has the greatest biodiversity among the world's high-altitude regions. Here, from the next-generation sequencing data, we obtained a known beta-coronavirus (beta-CoV) genome and a novel delta-coronavirus (delta-CoV) genome from faecal samples of 29 marmots, 50 rats and 25 birds in Yushu Tibetan Autonomous Prefecture, Qinghai Province, China in July 2019. According to the phylogenetic analysis, the beta-CoV shared high nucleotide identity with Coronavirus HKU24. Although the novel delta-CoV (MtCoV) was closely related to Sparrow deltacoronavirus ISU42824, the protein spike of the novel delta-CoV showed highest amino acid identity to Sparrow coronavirus HKU17 (73.1%). Interestingly, our results identified a novel host (Montifringilla taczanowskii) for the novel delta-CoV and the potential cross-species transmission. The most recent common ancestor (tMRCA) of MtCoVs along with other closest members of the species of Coronavirus HKU15 was estimated to be 289 years ago. Thus, this study increases our understanding of the genetic diversity of beta-CoVs and delta-CoVs, and also provides a new perspective of the coronavirus hosts.

PTEN Lipid Phosphatase Activity Enhances Dengue Virus Production through Akt/FoxO1/Maf1 Signaling
2021, 36(3): 412 doi: 10.1007/s12250-020-00291-6
Dengue virus (DENV) is an arthropod-borne viral pathogen and a global health burden. Knowledge of the DENV-host interactions that mediate virus pathogenicity remains limited. Host lipid metabolism is hijacked by DENV for virus replication in which lipid droplets (LDs) play a key role during the virus lifecycle. In this study, we reveal a novel role for phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in LDs-mediated DENV infection. We demonstrate that PTEN expression is downregulated upon DENV infection through post-transcriptional regulation and, in turn, PTEN overexpression enhances DENV replication. PTEN lipid phosphatase activity was found to decrease cellular LDs area and number through Akt/FoxO1/Maf1 signaling, which, together with autophagy, enhanced DENV replication and virus production. We therefore provide mechanistic insight into the interaction between lipid metabolism and the DENV replication cycle.
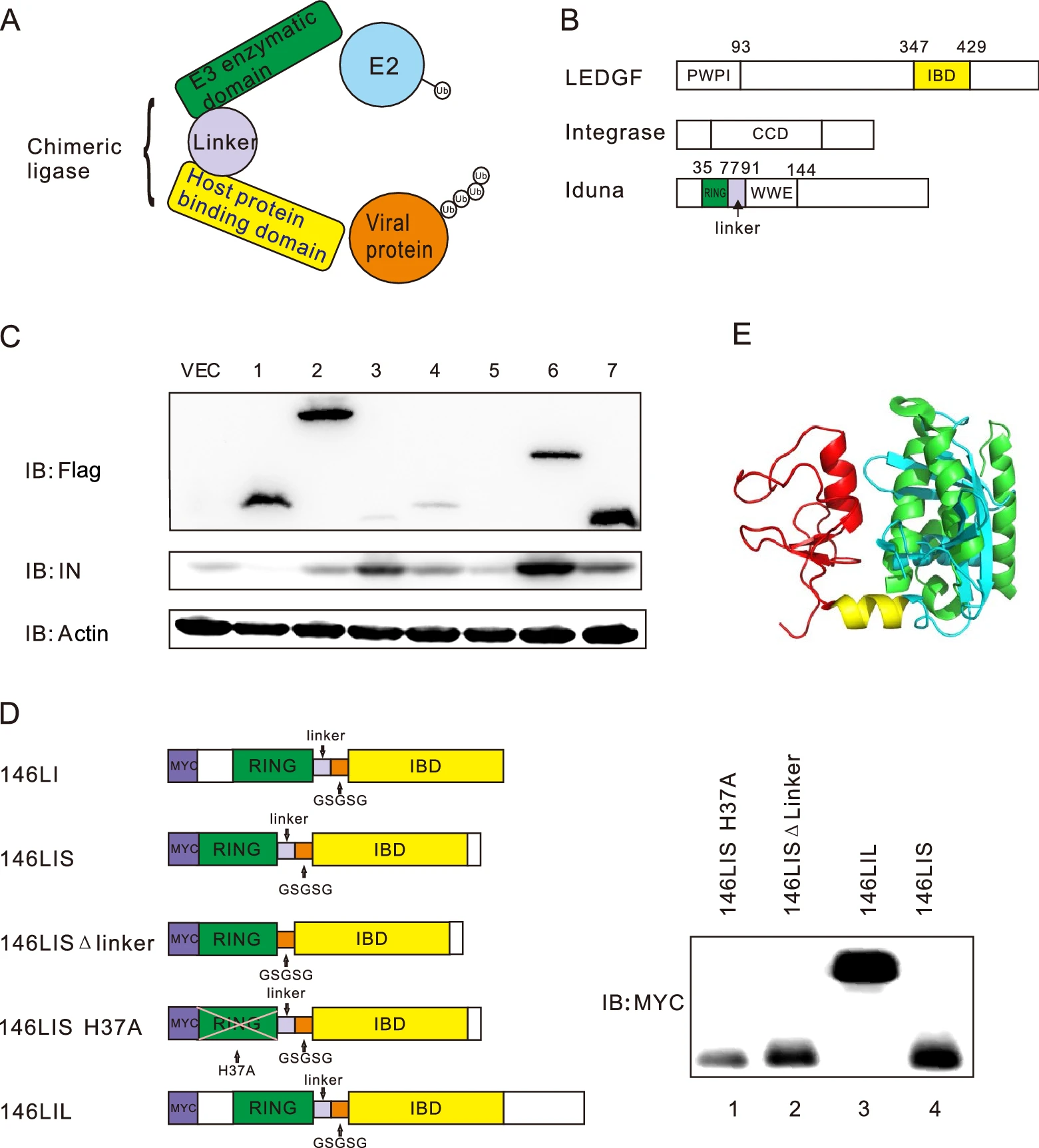
Suppression of HIV-1 Integration by Targeting HIV-1 Integrase for Degradation with A Chimeric Ubiquitin Ligase
2021, 36(3): 424 doi: 10.1007/s12250-020-00311-5
Human immunodeficiency virus (HIV) attacks human immune system and causes life-threatening acquired immune deficiency syndrome (AIDS). Treatment with combination antiretroviral therapy (cART) could inhibit virus growth and slow progression of the disease, however, at the same time posing various adverse effects. Host ubiquitin-proteasome pathway (UPP) plays important roles in host immunity against pathogens including viruses by inducing degradation of viral proteins. Previously a series of methods for retargeting substrates for ubiquitin-proteasome degradation have been successfully established. In this study, we attempted to design and construct artificial chimeric ubiquitin ligases (E3s) based on known human E3s in order to manually target HIV-1 integrase for ubiquitin proteasome pathway-mediated degradation. Herein, a series of prototypical chimeric E3s have been designed and constructed, and original substrate-binding domains of these E3s were replaced with host protein domains which interacted with viral proteins. After functional assessment screening, 146LI was identified as a functional chimeric E3 for HIV-1 NL4-3 integrase. 146LI was then further optimized to generate 146LIS (146LI short) which has been shown to induce Lys48-specific polyubiquitination and reduce protein level of HIV-1 NL4-3 integrase more effectively in cells. Lymphocyte cells with 146LIS knock-in generated by CRISPR/Cas-mediated homology-directed repair (HDR) showed remarkably decreased integration of HIV-1 NL4-3 viral DNAs and reduced viral replication without obvious cell cytotoxicity. Our study successfully obtained an artificial chimeric E3 which can induce Lys48-specific polyubiquitination and proteasome-mediated degradation of HIV-1 NL4-3 integrase, thus effectively inhibiting viral DNA integration and viral replication upon virus infection.
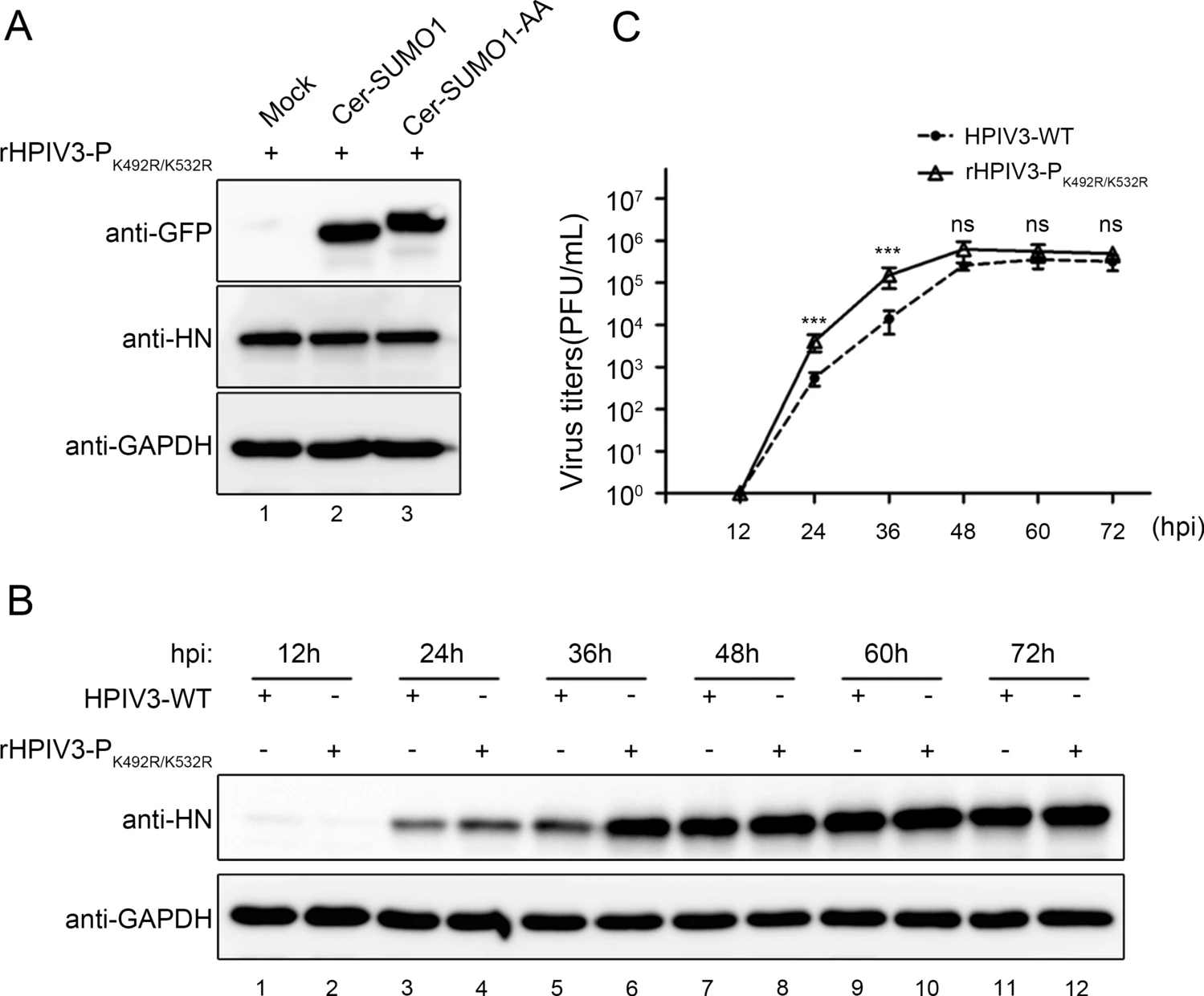
Sumoylation of Human Parainfluenza Virus Type 3 Phosphoprotein Correlates with A Reduction in Viral Replication
2021, 36(3): 438 doi: 10.1007/s12250-020-00314-2
Human parainfluenza virus type 3 (HPIV3), a member of the Paramyxoviridae family, can cause lower respiratory disease in infants and young children. The phosphoprotein (P) of HPIV3 is an essential cofactor of the viral RNA-dependent RNA polymerase large protein (L). P connects nucleocapsid protein (N) with L to initiate genome transcription and replication. Sumoylation influences many important pathways of the target proteins, and many viral proteins are also themselves sumoylated. In this study, we found that the P of HPIV3 could be sumoylated, and mutation of K492 and K532 to arginine (PK492R/K532R) failed to be sumoylated within P, which enhances HPIV3 minigenome activity. Biochemical studies showed that PK492R/K532R had no effect on its interactions with N, formation of homo-tetramers and formation of inclusion bodies. Finally, we found that incorporation of K492R/K532R into a recombinant HPIV3 (rHPIV3-PK492R/K532R) increased viral production in culture cells, suggesting that sumoylation attenuates functions of P and down-regulates viral replication.
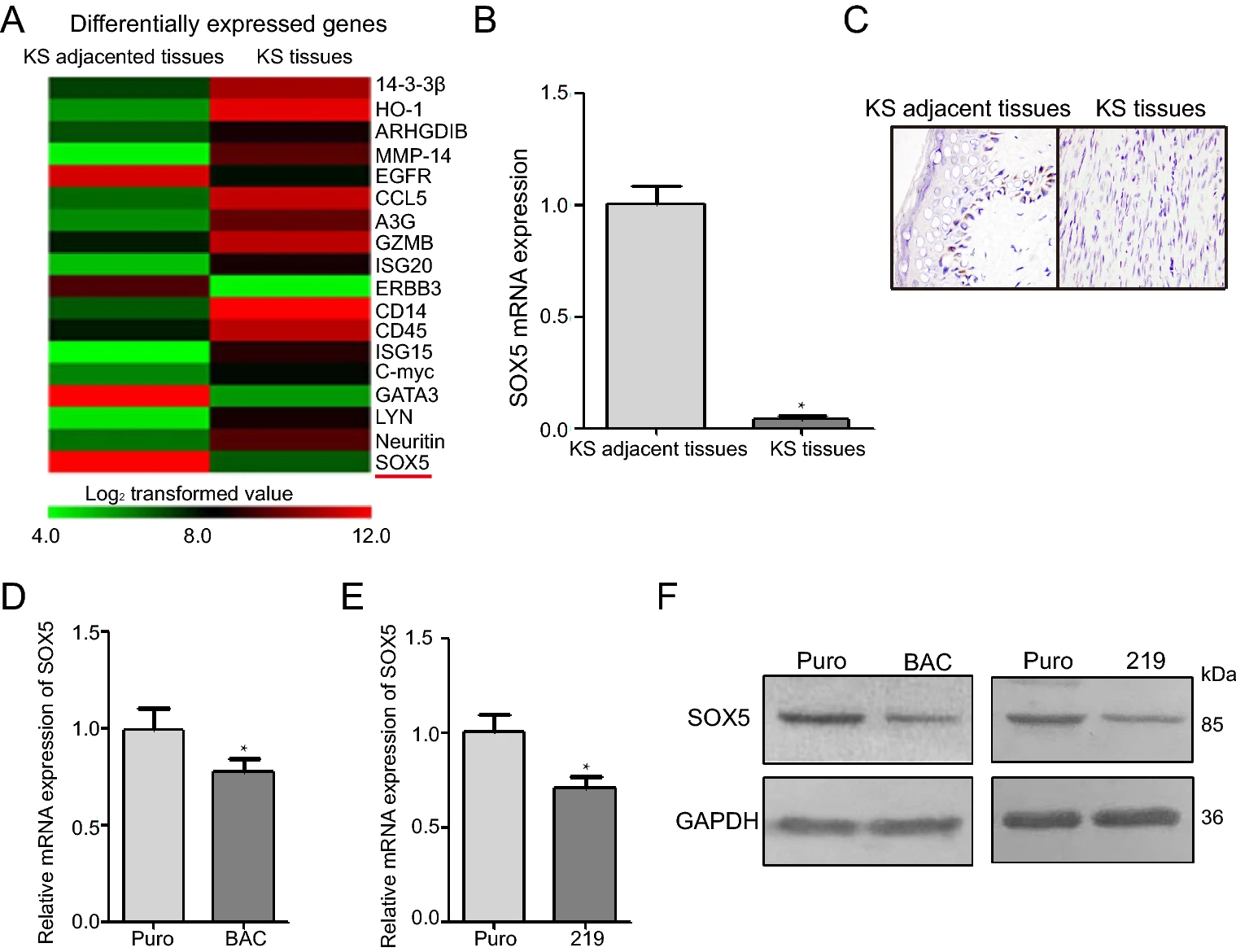
SOX5 Regulates Cell Proliferation, Apoptosis, Migration and Invasion in KSHV-Infected Cells
2021, 36(3): 449 doi: 10.1007/s12250-020-00313-3
Kaposi's sarcoma (KS) originates from vascular endothelial cells, with KS-associated herpesvirus (KSHV) as the etiological agent. SRY-box transcription factor 5 (SOX5) plays different roles in various types of cancer, although its role in KS remains poorly understood. In this study, we identified the role of SOX5 in KS tissues and KSHV-infected cells and elucidated the molecular mechanism. Thirty-two KS patients were enrolled in this study. Measurement of SOX5 mRNA and protein levels in human KS tissues and adjacent control tissues revealed lower levels in KS tissues, with KS patients having higher SOX5 level in the early stages of the disease compared to the later stages. And SOX5 mRNA and protein was also lower in KSHV-infected cells (iSLK-219 and iSLK-BAC) than normal cells (iSLK-Puro). Additionally, SOX5 overexpression inhibited cell proliferation and promoted apoptosis and decreased KSHV-infected cell migration and invasion. Moreover, we found that SOX5 overexpression suppressed the epithelial-to-mesenchymal transition of KSHV-infected cells. These results suggest SOX5 is a suppressor factor during KS development and a potential target for KS treatment.
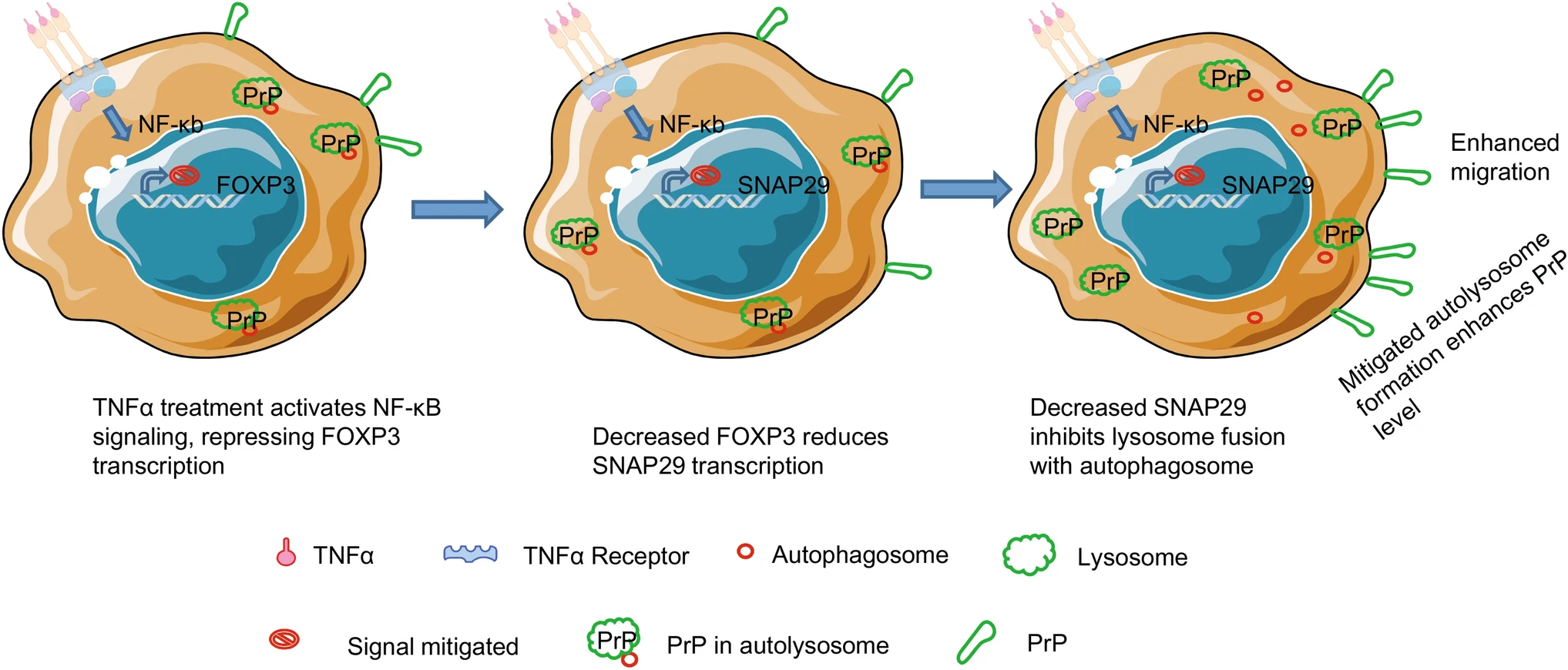
Tumor Necrosis Factor α Reduces SNAP29 Dependent Autolysosome Formation to Increase Prion Protein Level and Promote Tumor Cell Migration
2021, 36(3): 458 doi: 10.1007/s12250-020-00320-4
Tumor Necrosis Factor α (TNFα) is best known as a mediator of inflammation and immunity, and also plays important roles in tumor biology. However, the role of TNFα in tumor biology is complex and not completely understood. In a human melanoma cell line, M2, and a lung carcinoma cell line, A549, TNFα up-regulates prion protein (PrP) level, and promotes tumor cell migration in a PrP dependent manner. Silencing PRNP abrogates TNFα induced tumor cell migration; this phenotype is reversed when PRNP is re-introduced. Treatment with TNFα activates nuclear factor kappa B (NF-кB) signaling, which then mitigates autophagy by reducing the expression of Forkhead Box P3 (FOXP3). Down regulation of FOXP3 reduces the transcription of synaptosome associated protein 29 (SNAP29), which is essential in the fusion of autophagosome and lysosome creating autolysosome. FOXP3 being a bona fide transcription factor for SNAP29 is confirmed in a promoter binding assay. Accordingly, silencing SNAP29 in these cell lines also up-regulates PrP, and promotes tumor cell migration without TNFα treatment. But, when SNAP29 or FOXP3 is silenced in these cells, they are no longer respond to TNFα. Thus, a reduction in autophagy is the underlying mechanism by which expression of PrP is up-regulated, and tumor cell migration is enhanced upon TNFα treatment. Disrupting the TNFα-NF-кB-FOXP3-SNAP29 signaling axis may provide a therapeutic approach to mitigate tumor cell migration.
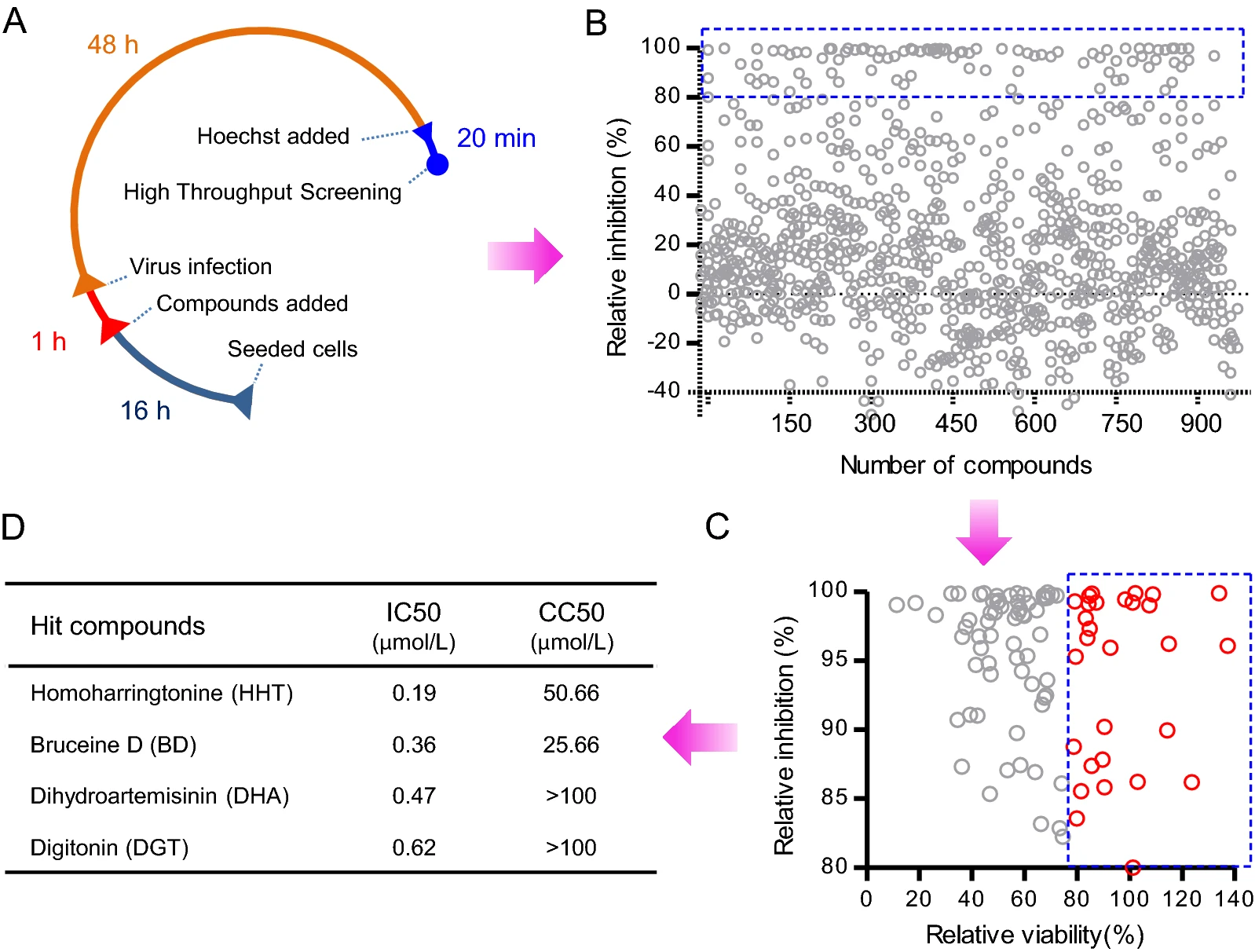
Generation of A Stable GFP-reporter Zika Virus System for High-throughput Screening of Zika Virus Inhibitors
2021, 36(3): 476 doi: 10.1007/s12250-020-00316-0
Zika virus (ZIKV) is associated with severe birth defects and Guillain-Barré syndrome and no approved vaccines or specific therapies to combat ZIKV infection are currently available. To accelerate anti-ZIKV therapeutics research, we developed a stable ZIKV GFP-reporter virus system with considerably improved GFP visibility and stability. In this system a BHK-21 cell line expressing DC-SIGNR was established to facilitate the proliferation of GFP-reporter ZIKV. Using this reporter virus system, we established a high-throughput screening assay and screened a selected plant-sourced compounds library for their ability to block ZIKV infection. More than 31 out of 974 tested compounds effectively decreased ZIKV reporter infection. Four selected compounds, homoharringtonine (HHT), bruceine D (BD), dihydroartemisinin (DHA) and digitonin (DGT), were further validated to inhibit wild-type ZIKV infection in cells of BHK-21 and human cell line A549. The FDA-approved chronic myeloid leukemia treatment drug HHT and BD were identified as broad-spectrum flavivirus inhibitors. DHA, another FDA-approved antimalarial drug effectively inhibited ZIKV infection in BHK-21 cells. HHT, BD and DHA inhibited ZIKV infection at a post-entry stage. Digitonin was found to have inhibitory activity in the early stage of viral infection. Our research provides an efficient high-throughput screening assay for ZIKV inhibitors. The active compounds identified in this study represent potential therapies for the treatment of ZIKV infection.
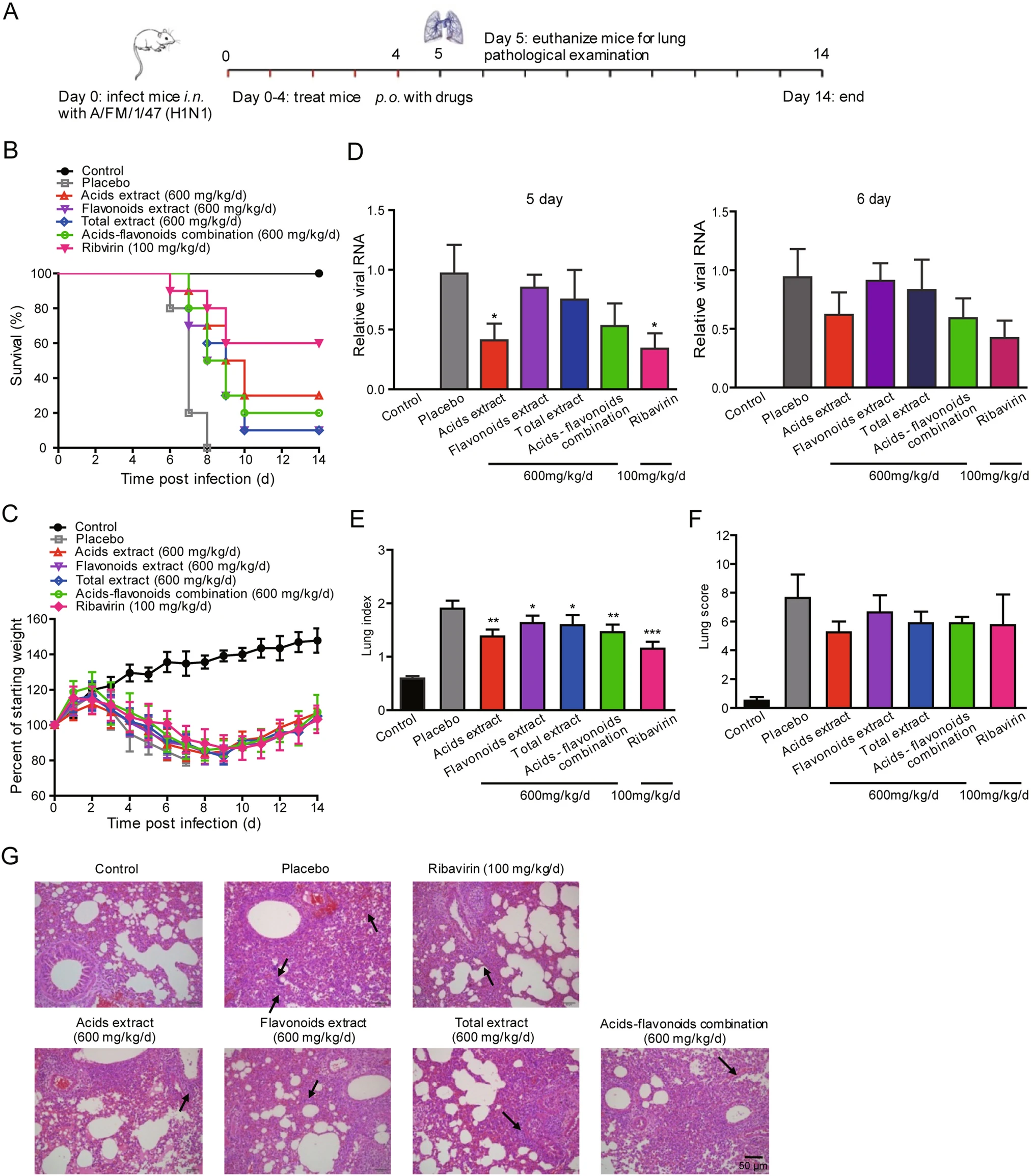
Inhibitory Activity of Honeysuckle Extracts against Influenza A Virus In Vitro and In Vivo
2021, 36(3): 490 doi: 10.1007/s12250-020-00302-6
Honeysuckle has been used in the treatment of influenza virus infection for thousands of years in China. However, its main active components and the functional mechanisms remain to be elucidated. Here, four honeysuckle extracts, including acids extract, flavonoids extract, total extract and acids-flavonoids mixture, were prepared to clarify the main active antiviral components. The cytopathic effect reduction assay showed that all the four extracts inhibited the replication of influenza viruses H1N1, H3N2 and the oseltamivir-resistant mutant strain H1N1-H275Y. The acids-flavonoids mixture had the strongest inhibitory effects in vitro with EC50 values of 3.8, 4.1, and >20 μg/mL against H1N1, H3N2 and H1N1-H275Y, respectively, showing competitive antiviral activity with oseltamivir and ribavirin. Honeysuckle acids extract also showed the most significant antiviral activity in vivo. Oral administration of the acids extract at a dosage of 600 mg/kg/d effectively alleviated viral pneumonia, maintained body weight and improved the survival rate to 30% of the mice infected with a lethal dose of H1N1. The results of time-of-drug addition experiment and neuraminidase (NA) inhibition assay showed that honeysuckle extracts had a broad-spectrum inhibitory effect against influenza virus NAs. The flavonoid extract showed the strongest inhibitory effect on the NA of influenza virus H7N9 with an IC50 of 24.7 μg/mL. These results suggested that these extracts might exert their antiviral activity by suppressing the release of influenza viruses. Briefly, our findings demonstrate that acids and flavonoids extracts of honeysuckle are the major antiviral active components, and the acids extract has the potential to be developed into an antiviral agent against influenza virus, especially for oseltamivir-resistant viruses.
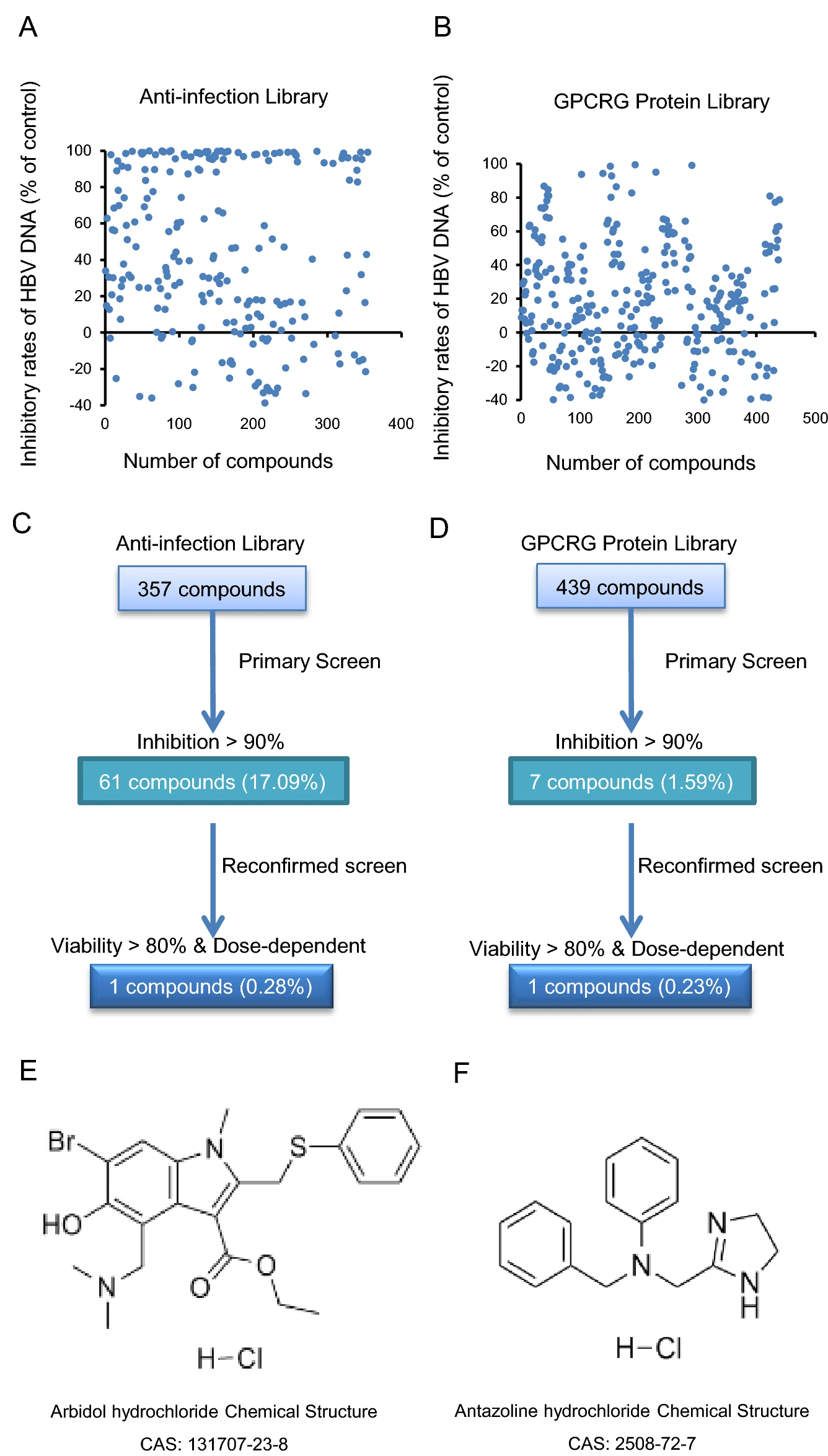
Repurposing of Antazoline Hydrochloride as an Inhibitor of Hepatitis B Virus DNA Secretion
2021, 36(3): 501 doi: 10.1007/s12250-020-00306-2
Hepatitis B virus (HBV) belongs to Hepadnaviridae family and mainly infects hepatocytes, which can cause acute or chronic hepatitis. Currently, two types of antiviral drugs are approved for chronic infection clinically: interferons and nucleos(t)ide analogues. However, the clinical cure for chronic infection is still rare, and it is a huge challenge for all researchers to develop high-efficiency, safe, non-tolerant, and low-toxicity anti-HBV drugs. Antazoline hydrochloride is a first-generation antihistamine with anticholinergic properties, and it is commonly used to relieve nasal congestion and in eye drops. Recently, an in vitro high-throughput evaluation system was constructed to screen nearly 800 compounds from the Food and Drug Administration (FDA)-approved Drug Library. We found that arbidol hydrochloride and antazoline hydrochloride can effectively reduce HBV DNA in the extracellular supernatant in a dose-dependent manner, with EC50 of 4.321 μmol/L and 2.910 μmol/L in HepAD38 cells, respectively. Moreover, the antiviral effects and potential mechanism of action of antazoline hydrochloride were studied in different HBV replication systems. The results indicate that antazoline hydrochloride also has a significant inhibitory effect on HBV DNA in the extracellular supernatant of Huh7 cells, with an EC50 of 2.349 μmol/L. These findings provide new ideas for screening and research related to HBV agents.
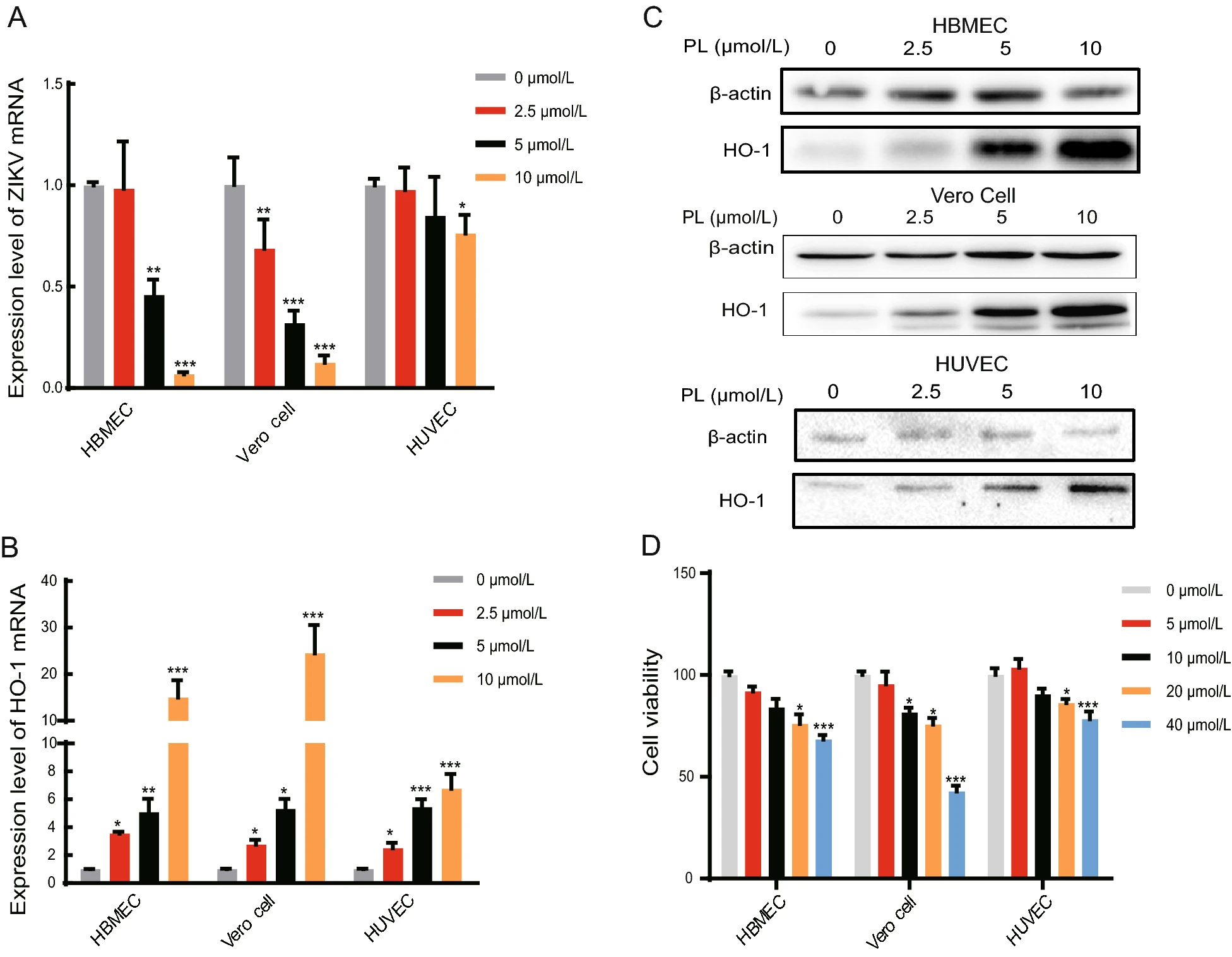
Piperlongumine Inhibits Zika Virus Replication In vitro and Promotes Up-Regulation of HO-1 Expression, Suggesting An Implication of Oxidative Stress
2021, 36(3): 510 doi: 10.1007/s12250-020-00310-6
Owing to the widespread distribution of mosquitoes capable of transmitting Zika virus, lack of clinical vaccines and treatments, and poor immunity of populations to new infectious diseases, Zika virus has become a global public health concern. Recent studies have found that Zika virus can continuously infect human brain microvascular endothelial cells. These cells are the primary components of the bloodɃbrain barrier of the cerebral cortex, and further infection of brain tissue may cause severe damage such as encephalitis and fetal pituitary disease. The present study found that a biologically active base, piperlongumine (PL), inhibited Zika virus replication in human brain microvascular endothelial cells, Vero cells, and human umbilical vein endothelial cells. PL also significantly increased heme oxygenase-1 (HO-1) gene expression, while silencing HO-1 expression and using the reactive oxygen species scavenger, N-acetylcysteine, attenuated the inhibitory effect of PL on Zika virus replication. These results suggest that PL induces oxidative stress in cells by increasing reactive oxygen species. This, in turn, induces an increase in HO-1 expression, thereby inhibiting Zika virus replication. These findings provide novel clues for drug research on the prevention and treatment of Zika virus.
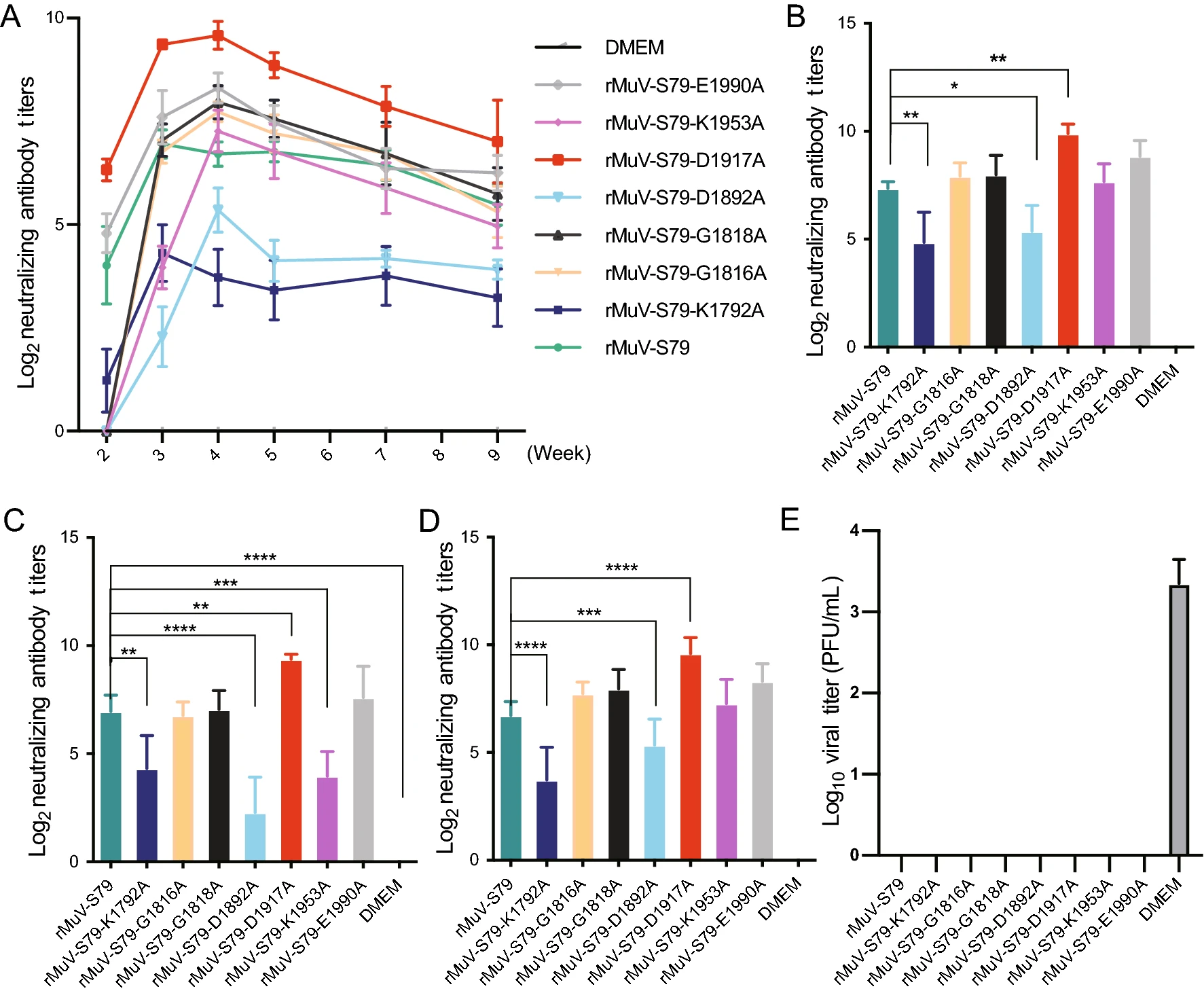
Development of Improved Mumps Vaccine Candidates by Mutating Viral mRNA Cap Methyltransferase Sites in the Large Polymerase Protein
2021, 36(3): 521 doi: 10.1007/s12250-020-00326-y
Although a live attenuated vaccine is available for controlling mumps virus (MuV), mumps still outbreaks frequently worldwide. The attenuated MuV vaccine strain S79 is widely used in mumps vaccination in China, but still with many shortcomings, among which the most prominent are the side effects and decreased immunity. Therefore, there is a need to further improve the safety and efficacy of the current MuV vaccine. In the present study, we further attenuated MuV S79 vaccine strain by inhibiting viral mRNA methyltransferase (MTase). We generated a panel of eight recombinant MuVs (rMuVs) carrying mutations in the MTase catalytic site or S-adenosylmethionine (SAM) binding site in the large (L) polymerase protein. These rMuVs are genetically stable and seven rMuVs are more attenuated in replication in cell culture and five rMuVs are more attenuated in replication in lungs of cotton rats compared with the parental vaccine strain S79. Importantly, cotton rats vaccinated with these seven rMuV mutants produced high levels of serum neutralizing antibodies and were completely protected against challenge with a wild-type MuV strain (genotype F). Therefore, our results demonstrate that alteration in the MTase catalytic site or SAM binding site in MuV L protein improves the safety or the immunogenicity of the MuV vaccine and thus mRNA cap MTase may be an effective target for the development of new vaccine candidates for MuV.
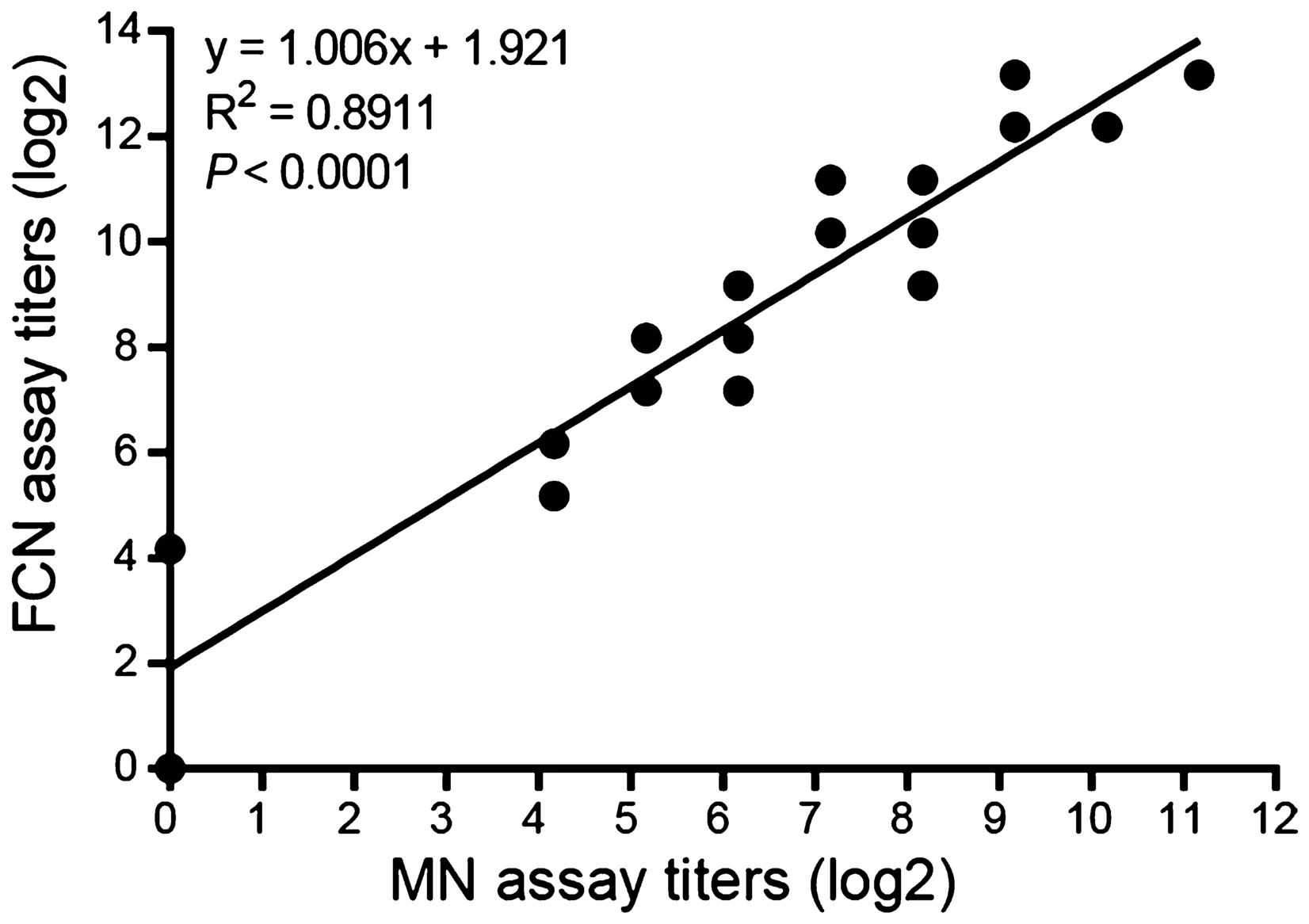
A Sensitive and High-Throughput Flow Cytometry-Based Assay for Measuring Antibody Neutralization of Human Adenovirus Type 3
2021, 36(3): 537 doi: 10.1007/s12250-020-00295-2
The assessment of neutralization activity is an important step in the evaluation of neutralizing antibodies (NAbs). The traditional methods for measuring the antibody neutralization of human adenovirus type 3 (HAdV-3) are the microneu-tralization (MN) assay, which has insufficient sensitivity, and the plaque reduction neutralization test (PRNT), which is not suitable for high-throughput screening. Herein, we describe the development of a flow cytometry-based neutralization (FCN) assay for measuring the neutralization of sera, cell culture supernatants, and chimeric antibodies against HAdV-3 on the basis of a recombinant HAdV-3 (rHAdV-3) construct expressing the enhanced green fluorescent protein (EGFP). For flow cytometry-based assays, the optimal cell confluence was determined as 90%, and the virus was titrated using the assay. The established FCN assay follows the percentage law and an optimal MOI of not less than 5×10-4 was determined by using a purified chimeric antibody. In addition, comparison of the anti-HAdV-3 NAb titers of 72 human serum samples by the MN and FCN assays, showed that both assays correlated strongly with each other. Our FCN assay was an improvement over the MN assay because the observation period was reduced from 3 to 1 days and data analysis could be performed objectively and robotically. Importantly, the newly established FCN assay allows measurement of the neutralization activity of chimeric antibodies expressed in cell culture supernatants. Thus, this sensitive and high-throughput FCN assay is a useful alternative to the MN assay for measuring the antibody neutralization of HAdV-3 and for screening anti-HAdV-3 NAbs in cell culture supernatants.

Molecular Characterization of Human Respiratory Adenoviruses Infection in Xining City, China In 2018
2021, 36(3): 545 doi: 10.1007/s12250-020-00282-7
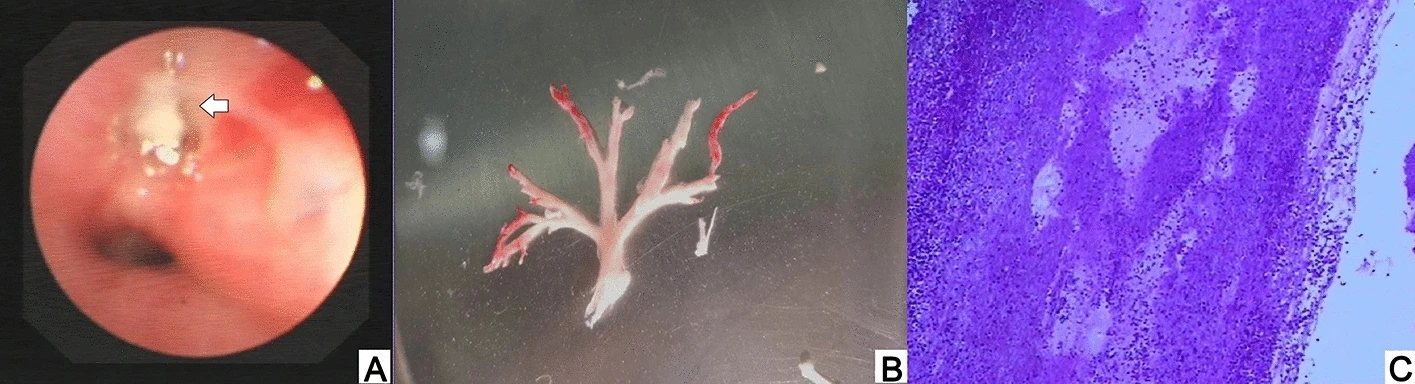
Clinical Characteristics of Human Adenovirus Plastic Bronchitis in 10 Pediatric Cases: A Retrospective Study of Seven Years
2021, 36(3): 550 doi: 10.1007/s12250-021-00394-8
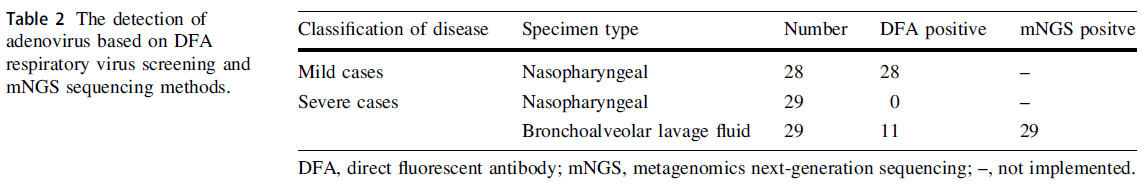
Retrospective Study of an Adenovirus Pneumonia Outbreak in Shenzhen in 2017
2021, 36(3): 555 doi: 10.1007/s12250-021-00393-9

Broad Cell Tropism of SADS-CoV In Vitro Implies Its Potential Cross-Species Infection Risk
2021, 36(3): 559 doi: 10.1007/s12250-020-00321-3
RETRACTED ARTICLE: Human H9N2 Avian Influenza Infection: Epidemiological and Clinical Characterization of 16 Cases in China
2021, 36(3): 564 doi: 10.1007/s12250-020-00248-9